- Visibility 43 Views
- Downloads 7 Downloads
- DOI 10.18231/j.ijceo.2022.088
-
CrossMark
- Citation
Choroidal neovascularization caused by angioid streaks: seven-year follow-up
Introduction
Angioid streaks are red-brown or red-grey irregular lines of variable size and shape radiating from the peripapillary region for varying distances into the fundus, lying under the retinal vessels.[1] They are caused by ruptures of calcified Bruch’s membrane and are later often altered in appearance by proliferative changes along or within them.[2]
The most common and sight threating complication of angioid streaks is choroidal neovascularization (CNV), which is often bilateral but asymmetric.[3] This complication becomes challenging for ophthalmologists and deteriorates the prognosis of disease when it affects the macular region.[4]
In the therapy of CNV caused by angioid streaks, intravitreal anti vascular endothelial growth factor (VEGF) drugs are currently the treatment of choice, and they have been proven to improve or stabilize visual acuity.[3], [5], [6]
In this case intravitreal injection of bevacizumab and aflibercept was used as the treatment of choice.
Case Presentation
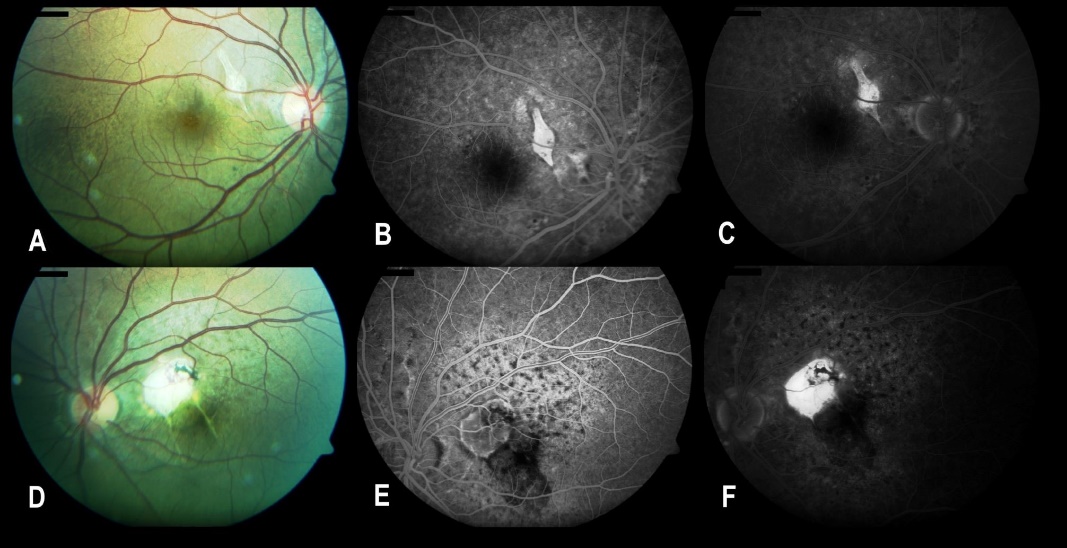
A 29-year-old man presented in 2014 with complaints of impaired vision in his left eye. The best corrected visual acuity (BCVA) was 20/32 in his right eye and 20/2000 in his left eye. Intraocular pressure measurements and the slit lamp biomicroscopic examination of anterior segments in both eyes were found to be normal. Dilated fundus examination revealed bilateral subretinal red-brown lines extending from peripapillary area to periphery of fundus and macula. There were chorioretinal lesions with surrounding subretinal fluid in the superior nasal part of macula in both eyes. This lesion defined as choroidal neovascular membrane (CNVM) extended to parafovea and fovea in his left eye. Some intraretinal hemorrhages at the inferior border of the subretinal fluid area were also seen in the left eye. Optical coherence tomography (OCT) examinations of the macula and the lesion ([Figure 1]) showed high reflective choroidal neovascular membrane and subretinal fluid in both eyes. Central macular thickness was 253 µm in the right eye and 822 µm in the left eye. The fundus fluorescein angiography (FFA) examination demonstrated an early macular hyperfluorescent lesion with late staining and hyperfluorescent linear streaks that expanded from the peripapillary region to the periphery in both eyes. The mottled hyperfluorescence was seen in early phase of angiogram ([Figure 1]). The examinations confirmed the final diagnosis as angioid streaks with the complication of choroidal neovascularization. Intravitreal bevacizumab therapy was initiated with three consecutive injections in the right eye and two consecutive injections in the left eye at one-month intervals.

After injections the BCVA increased to 20/25 in the right eye, 20/800 in the left eye. After first injections patient was admitted to the FFA examination again. There was not any active leakage from hyperfluorescent lesion in the right eye, whereas the FFA showed leakage of dye from the CNVM in the left eye ([Figure 2]). Intravitreal bevacizumab injection was performed in the left eye. After 3 total intravitreal injections of bevacizumab, therapy was discontinued in the left eye due to macular scar formation, no active process and stable BCVA (it was 20/2000 after third injection).
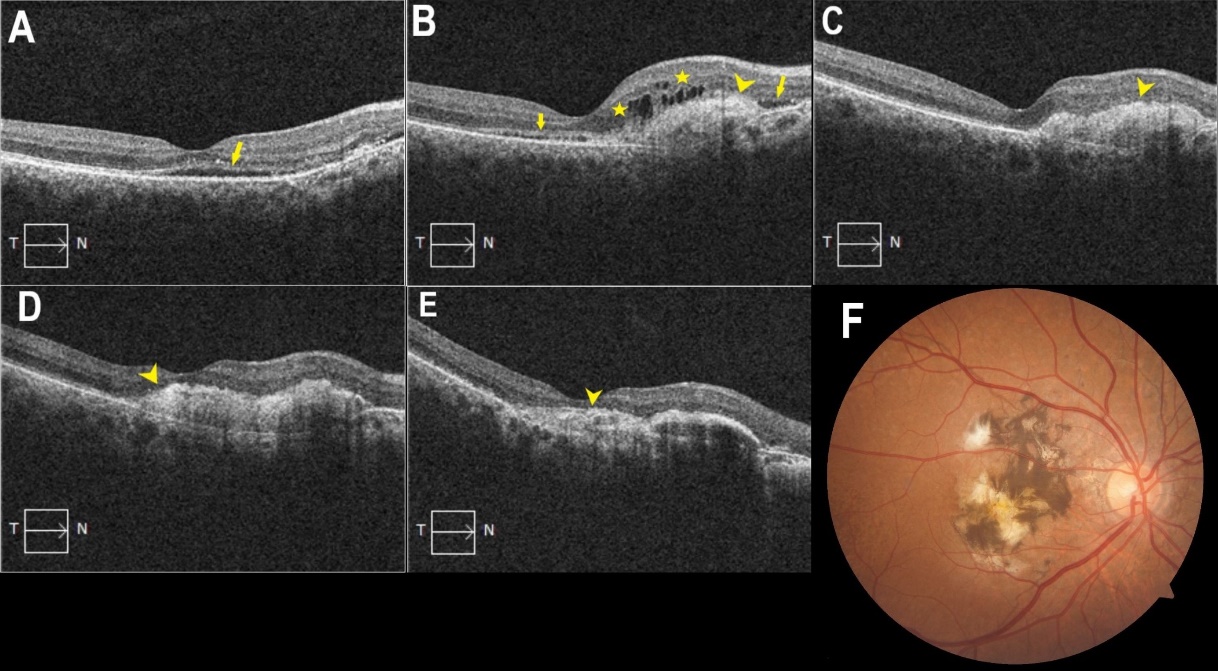
Repetitive intravitreal bevacizumab injections were continued in his right eye and were performed due to membrane activity, monitored by OCT images ([Figure 3]). It should be noted that during the covid-19 pandemic, the patient did not visit the clinic for 10 months. This led to the progression of the subretinal membrane and a significant decrease in visual acuity in the right eye. After a follow-up period of 7 years, the patient received 22 intravitreal bevacizumab and 4 intravitreal aflibercept injections in the right eye and 3 intravitreal bevacizumab injections in the left eye. No injection-related adverse events were reported. At last visit the BCVA was 20/400 in his right eye and 20/2000 in the left eye.
Discussion
Angioid streaks are dark reddish-brown irregular lines radiating from the optic disc toward the retinal periphery. They are often bilateral and indicate crack-like breaks of a calcified and fragile Bruch membrane.[3]
In up to 50% cases, angioid streaks can be idiopathic.[7] Angioid streaks are associated with systemic conditions in more than 50 percent of patients. Pseudoxanthoma elasticum, Paget disease, Ehlers–Danlos syndrome, Marfan syndrome, hemoglobinopathies, and hypercalcemia are common systemic associations.[3] In this patient, we thought of pseudoxanthoma elasticum (PXE) due to specific changes in the neck area and mottled fundus or peau d’orange appearance (mottled hyperfluorescence in angiogram) ([Figure 1], [Figure 2]). The mottled fundus, also known as "peau d'orange," is the pathognomonic feature of PXE in the fundoscopic examination.[1]
Angioid streaks cause some complications. They include subretinal bleed caused by choroidal rupture after little trauma, choroidal neovascularization, foveal atrophy, or thinning.[7] The most common and significant complication of angioid streaks is choroidal neovascularization, and it occurs mainly bilaterally and asymmetrically.[3] It is shown that 70-86% of patients with angioid streaks develop CNVM.[8] If left untreated, it can lead to severe vision loss in middle-aged working adults.[3]
The patient was diagnosed with type II CNV by OCT imaging as the neovascular vessels were enlarging in the subretinal space between the retinal pigment epithelium and the neurosensory retina, as opposed to type I CNV, in which the vessels grow below the retinal pigment epithelium.[9], [10] The most prominent form is type II CNV, whereas type I and mixed (type I and type II) forms can occur in up to 16 percent of eyes.[11]
Anti-VEGF is the treatment of choice in therapy of CNV caused by angioid streaks, as these agents have been shown to improve or stabilize the visual acuity.[3], [5], [6] Battaglia et al. reported a 33% increase and 46.6% stabilization in visual acuity, with the mean number of injections 2.5.[12] Alagoz C. et al. demonstrated an improvement or stabilization in visual acuity in 65% of eyes with CNV due to angioid streaks, whereas visual acuity worsened in 35% of eyes.[13]
In our case, the patient had 22 intravitreal bevacizumab and 4 intravitreal aflibercept injections in the right eye and 3 intravitreal bevacizumab injections in the left eye after a 7-year follow-up period. The number of injections were determined based on the clinical and imagining findings of the patient. The patient did not visit the clinic for 10 months during the COVID-19 pandemic. This led to the progression of the subretinal membrane and a significant decrease in visual acuity in the left eye.
Thushanthi et al. showed in their case series, maximum follow-up of 10 years, anti-VEGF therapy is safe and effective in stabilizing and slowing progression of the disease. They started treatment with bevacizumab, then switched to ranibizumab or aflibercept in 66 eyes of 52 patients. Mean BCVA at baseline was 6/19 (62 ETDRS letters). At 2 years follow-up the mean BCVA was 6/15 (>5 ETDRS letters). After 5 or greater years of follow-up, there was average loss of ETDRS letters from baseline of 3.3 letters. Subfoveal CNV showed worse outcome in comparison with extrafoveal and juxtafoveal CNV.[6]
Similarly, in our case, the BCVA in the right eye was 20/50 after two years of follow-up. After 3 years of follow-up the BCVA was 20/63, after 4 years it decreased to 20/200. After 5 or greater years of follow up, the BCVA decreased to 20/400 and stayed stable.
The frequency of anti-VEGF treatment remains unclear. Some studies demonstrated that the frequent use of anti-VEGF agents in patients with angioid streaks may cause atrophic changes in the retinal pigment epithelium and neurosensory retinal layers because the breaks of the Bruch membrane allow the drug to easily enter the choriocapillaris.[14] In our case after the initial loading dose the intravitreal bevacizumab injections were performed due to membrane activity, monitored by OCT images.
Conclusion
Rapid progression and high recurrence rates of CNV due to angioid streaks indicates poorer prognosis. Anti-VEGF therapy can help to stabilize the disease, however, a high rate of recurrence is inevitable. Long-term follow-up of the patient shows gradual vision worsening over time. Furthermore, there is a significant vision loss observed after 5-7 years of follow-up. One of the possible reasons for the worsening of the process in this case may be delayed visits to the clinic due to the COVID-19 pandemic.
Source of Funding
No source of funding.
Conflicts of Interest
No conflicts of interest.
References
- JA Shields, JL Federman, TL Tomer, WH Annesley. Angioid streaks. I. Ophthalmoscopic variations and diagnostic problems. Br J Ophthalmol 1975. [Google Scholar]
- FH Verhoeff. Histological findings in a case of angioid streaks. Br J Ophthalmol 1948. [Google Scholar]
- I Chatziralli, G Saitakis, E Dimitriou, A Chatzirallis, S Stoungioti, G Theodossiadis. ANGIOID STREAKS: A Comprehensive Review from Pathophysiology to Treatment. Retina 2019. [Google Scholar]
- I Georgalas, D Papaconstantinou, C Koutsandrea, G Kalantzis, D Karagiannis, G Georgopoulos. Angioid streaks, clinical course, complications, and current therapeutic management. Ther Clin Risk Manag 2009. [Google Scholar]
- M Gliem, RP Finger, R Fimmers, CK Brinkmann, FG Holz, PC Issa. Treatment of choroidal neovascularisation due to angioid streaks: a comprehensive review. Retina 2013. [Google Scholar]
- T Ramakrishnan, S Chandra, S Sivaprasad. Long-term follow-up of management of choroidal neovascularisation secondary to angioid streaks with intravitreal anti-vascular endothelial growth factor. Eye 2021. [Google Scholar]
- K Tripathy, JM Quint. . Angioid Streaks [Internet] 2022. [Google Scholar]
- JI Lim, NM Bressler, MJ Marsh, SB Bressler. Laser treatment of choroidal neovascularization in patients with angioid streaks. Am J Ophthalmol 1993. [Google Scholar]
- HE Grossniklaus, WR Green. Choroidal neovascularization. Am J Ophthalmol 2004. [Google Scholar]
- Y Nakano, K Kataoka, J Takeuchi, A Fujita, H Kaneko, H Shimizu. Vascular maturity of type 1 and type 2 choroidal neovascularization evaluated by optical coherence tomography angiography. PLoS One 2019. [Google Scholar]
- O Gal-Or, C Balaratnasingam, KB Freund. Optical coherence tomography angiography findings of choroidal neovascularization in pseudoxanthoma elasticum. Int J Retina Vitreous 2015. [Google Scholar]
- M Battaglia Parodi, P Lacono, C Laspina, L Berchicci, F Scotti, A Leys. Intravitreal bevacizumab for nonsubfoveal choroidal neovascularization associated with angioid streaks. Am J Ophthalmol 2014. [Google Scholar]
- C Alagöz, N Alagöz, A Özkaya, U Çelik, MF Turan, AT Yazici. Intravitreal bevacizumab in the treatment of choroidal neovascular membrane due to angioid streaks. Retina 2015. [Google Scholar]
- RP Finger, PC Issa, P Schmitz-Valckenberg, FG Holz, HN Scholl. Long-term effectiveness of intravitreal bevacizumab for choroidal neovascularization secondary to angioid streaks in pseudoxanthoma elasticum. Retina 2011. [Google Scholar]
