- Received November 30, -0001
- Accepted November 30, -0001
- Publication December 22, 2020
- Visibility 22 Views
- Downloads 1 Downloads
- DOI 10.18231/j.ijceo.2020.120
-
CrossMark
- Citation
Safety and efficacy of combined moxifloxacin and dexamethasone eye drops as prophylaxis in cataract surgery patients
- Author Details:
-
Nirupama Damarla *
-
G Satyanarayana Reddy
-
T Sanjay Reddy
-
B Hymavathi
-
Nasreen K
Introduction
Cataract surgery is the most common ophthalmic surgical procedure in India. Endophthalmitis following cataract surgery although very rare is one of the most serious, sight-threatening complications of ocular surgery. Antibiotic prophylaxis is a common preventive measure. The theoretical goal of topical antibiotic prophylaxis is to reduce the conjunctival bacterial load, thereby lowering the risk of intraocular contamination either intraoperatively or postoperatively. Topical antibiotics are frequently used for up to 1–2 weeks postoperatively until the incision fully heals and should not be tapered as this would encourage emergence of resistant organisms.[1] Moxifloxacin a fourth-generation fluoroquinolone achieves bactericidal levels at least 10 times the MIC of the most resistant bacteria for a limited time period, but because of its potent dose-dependent activity even at low injection concentrations, it remains bactericidal for a much longer duration.[2]
Topical corticosteroids such as Prednisolone and dexamethasone have been used for many years to control ocular inflammation resulting from cataract removal.[3], [4], [5] Conventional postoperative therapy for cataract surgery includes antibiotic eye drops for 2 weeks and steroid eye drops starting with 6 times a day tapered over 6 weeks[6] for an uneventful cataract surgery.
The antibiotic-plus-anti-inflammatory protocol is credited with keeping infectious and inflammatory complications at a low rate[7], [8] as inadequately controlled inflammation increases the risk of postoperative pain, edema, erythema, anterior chamber cells and flare, secondary glaucoma, posterior synechia, and, potentially, cystoid macular edema (CME).[9], [10], [11], [12], [13]
A fixed-combination eye drops helps in decreasing the cost and also improves patient compliance due to convenience in dosing and application.[14] The combination of moxifloxacin 0.5%/dexamethasone 0.1% (Vigadexa, Alcon Laboratories, Inc., Fort Worth, Tex, USA) eye drops are available. The safety and efficacy of this drug in various ophthalmic surgeries has been evaluated.[4], [7] The purpose of this study was to evaluate the safety and efficacy of the combination moxifloxacin 0.5%/dexamethasone 0.1% eye drops formulation in the prevention of postoperative inflammation and infection following phacoemulsification in south Indian patients.
Materials and Methods
A total of 97 patients fulfilling the eligibility criteria were recruited in the study. The study protocol was approved by institutional review board and informed consent was obtained from each patient prior to participation in the study.
Patients (>18 years) diagnosed with cataract underwent clear corneal phacoemulsification with posterior chamber IOL implantation.
Inclusion criteria
1. ≧18 years of age.
Able to sign an informed consent and complete all required visits.
Intends to undergo cataract surgery with posterior chamber Intraocular lens (IOL).
Intraocular Pressure (IOP ≦ 20 mmHg.
Exclusion criteria
Uncontrolled glaucoma or IOP.
Use of ocular medication during or within 30 days prior to the enrollment.
Use of steroid during the study or within 14 days prior to enrollment.
Treatment protocol
All the patients were instructed to instill moxifloxacin 0.5%/dexamethasone 0.1% (Vigadexa) eye drops four times a day starting from one day prior to undergoing cataract surgery to 2 weeks postoperatively. Povidone-iodine 5% solution placed in the inferior conjuctival fornix immediately before surgery within the preoperative preparation to significantly reduce the conjunctival bacterial flora.[15]
The patients underwent clear corneal (2.8 mm) phacoemulsification with single piece aspheric acrylic posterior chamber IOL implantation. 3-mL bottle of moxifloxacin (Vigamox) was diluted with 7 mL of BSS to achieve a concentration of 150 mcg/0.1mL, of which 0.3 to 0.4 mL was injected into the anterior chamber at the end of the surgery.
Patients were seen postoperatively on day 1, 7 and 15. In each visit they were evaluated for visual acuit, eye pain, signs or symptoms of ocular surface inflammation, presence of cells in the anterior chamber, and intraocular pressure elevation. The visual acuity was determined with snellens chart. The IOP was measured using Goldmann Applanation Tonometer. Slit-lamp biomicroscopy under high magnification was used to count the number of cells in the anterior chamber; a score of 0 ≤ 5 cells; 1 = 5–10 cells; 2 = 11–20 cells; 3 = 21–50 cells; and 4 ≥ 50 cells. Any visible sign of active ocular inflammation – redness, swelling, tearing, or discharge – was documented. The patients were asked to subjectively rank their eye pain on a 5-point scale, from 0 = none to 5 = severe. At each visit patients were also questioned regarding compliance with their treatment and occurrence of any adverse event in the interim since their previous examination. At the last visit (day 15), dilated fundoscopy was performed to document the status of the vitreous, retina, macula, choroid, and optic nerve. They were instructed to discontinue the medications and were discharged from the study.
Results
Ninety three patients were enrolled in the study. Of them 39 were males (49.1%) and 54 were females(58.1%). All of them were from South India. The mean age was 60.13 years ± 7.5 years (SD). Six were lost to followup, and three were shifted to moxifloxacin (Vigamox) and prednisolone acetate (Pred Forte) on first postoperative day because of severe ocular inflammation. Three patients were advised to use Sodium chloride 5% eye drops 4 times a day for 1 week from first postoperative day due to striate keratopathy (SK).
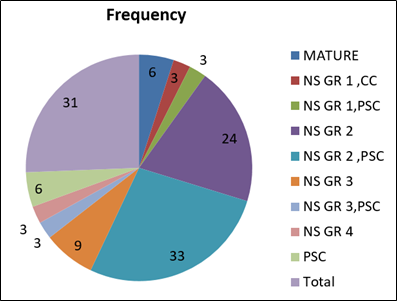
The inflammatory response was increased in the first week after surgery. It gradually declined by day 15. On first postoperative day 77.3% of patients had AC cells grade 3–2, while 19.3% had grade 1 AC cells. By 7th postoperative day 31.03% had grade 1 AC reaction and 68.96% had Grade 0 cells. By the end of the study i.e, on 15th postoperative day 89.65% had grade 0 AC cells and 31.03% had grade 1 cells. ([Figure 1]).
Ocular pain score on the first postoperative day was Grade 4 in 9.6% patients. It was Grade 3 and 2 in 48.38% and 41.93%. By 7th postoperative day 22.5% patients had Grade 2 ocular pain and 77.41% had grade 1. By 15th day ocular pain subsided with 12.9% had grade 1 ocular pain and 87.09% had grade 0 pain.
Signs of active inflammation in the eyelid and conjunctiva as evidenced by lid edema and conjuctival congestion significantly decreased from first postoperative day to 15th postoperative day. At the end of the study, signs of inflammation in the conjunctiva were seen only in 2 eyes.
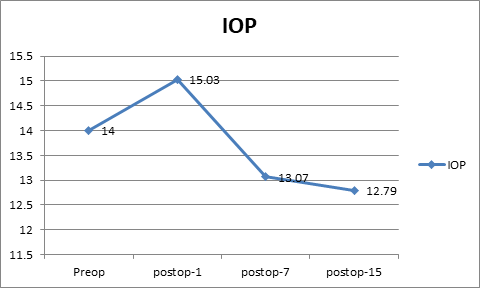
The mean IOP preoperatively was 14.00±3.8 mm Hg. The mean IOP on first and 7th postoperative was 15.03 ± 3.8 mm Hg and 13.07±2.7 mmHg respectively. On postoperative day15 the mean IOP was 12.79±2.5 mm Hg. ([Figure 3]) Fundus examination was done on all the patients at the end of the study which was normal. No drug allergies or drug-related adverse event was reported.
Discussion
In our study we have assessed the safety and efficacy of combined moxifloxacin 0.5%/dexamethasone 0.1% eyedrops (Vigadexa) in preventing postoperative inflammation and infection following phacoemulsification in south Indian patients. The main criterion of effectiveness was the extent of anterior chamber cell drop-off.
At the end of the study, i.e, by 15th postoperative day cells in the AC less than 5 was found in 89.65% of eyes. These figures are similar to the study done by Freitas LL et al[16] and by Cesar Ramon G. Espiritu et al.[14] Patients with AC reaction greater than grade 0 were given Loteprednol eye drops 4 times a day for 1 week after discontinuing Vigadexa eye drops.
On the 1st postoperative day, 77.3% of the patients had grade 2-3 AC reaction. By 7th postoperative day the grading of AC reaction decreased to grade 1in 31.03% and grade 0 in 68.96%. Similar postoperative AC reaction pattern were observed by the investigators in cases where separate antibiotic and corticosteroid eye drops were given after cataract surgery.
The postoperative inflammatory pattern in the eyelid/conjunctiva and cornea was consistent with previous observations on patients with postoperative use of Moxifloxacin eye drops and Prednisolone eye drops separately. All these were reflective of the mild ocular changes expected to occur as a result of surgery.
In our study, no surgery-related infection has occurred. However, the rarity of the event and the size of the study population did not allow us to make statistically significant conclusions about the effectiveness of the medication in preventing POE(Postoperative Endophthalmitis). Prior to our study, the efficacy of the fixed combination had already been established in a study by Freitas et al. in a Brazilian population.[16] In this randomized, parallel-group trial, the combination moxifloxacin 0.5%/dexamethasone 1% was as effective in preventing infection and controlling inflammation postoperatively compared to when its individual components were administered concurrently. Another study was done Cesar Ramon G. Espiritu et al. in Asian population proving the efficacy of the fixed combination. The safety parameters taken as criteria were of ocular pain, and intraocular pressure. The eyes that received the combined moxifloxacin/dexamethasone eye drops had identical results to those dosed conventionally with moxifloxacin and dexamethasone from separate bottles.[17], [18], [19]
We have also evaluated patient compliance of the fixed-combination preparation of moxifloxacin 0.5% and dexamethasone 0.1% in our study. The drug was well tolerated by patients; None of them have reported any discomfort during or immediately after its application confirming previous studies.[17], [18], [19], [20], [21]
Good patient compliance was determined by oral inquiry during follow-up consultations. Compliance can be attributed to the tolerability profile of the drug and ease of administration. Patients will comply with instilling an eye drop that does not sting, burn, cause redness, or blur vision. Furthermore, applying less number of drops makes it easier for patients to remember and adhere to the dosing regimen. With a combination preparation, patients no longer have to wait a minimum of five minutes to instill a drop from a separate medication to prevent a wash-out effect.
Conclusion
The fixed-combination of moxifloxacin 0.5% dexamethasone 0.1% eyedrops was found to be effective in preventing infection and minimizing inflammation following cataract surgery in south Indian population.
Fixed drug combinations are advantageous in developing countries especially where the socio-economic conditions and also the compliance of patients play an important role.
Source of Funding
None.
Conflict of Interest
None.
References
- A Haripriya. Antibiotic prophylaxis in cataract surgery – An evidence-based approach. Indian J Ophthalmol 2017. [Google Scholar] [Crossref]
- S A Arshinoff, M Modabber. Dose and administration of intracameral moxifloxacin for prophylaxis of postoperative endophthalmitis. J Cataract Refract Surg 2016. [Google Scholar] [Crossref]
- S M El-Harazi, R M. Feldman. Control of intra-ocular inflammation associated with cataract surgery. Curr Opin Opthalmol 2001. [Google Scholar] [Crossref]
- J N Simone, M M Whitacre. Effects of anti-inflammatory drugs following cataract extraction. Currt Opin Opthalmol 2001. [Google Scholar] [Crossref]
- C G Laurell. Effects of dexamethasone, diclofenac, or placebo on the inflammatory response after cataract surgery. Br J Ophthalmol 2002. [Google Scholar] [Crossref]
- A Haripriya, Z R Baam, R D Ravindran. Postoperative cataract care: the Aravind perspective. Community Eye Health J 2016. [Google Scholar]
- C P Herbort, A Jauch, P Othenin-Girard, J J Tritten, M Fsadni. Diclofenac drops to treat inflammation after cataract surgery. Acta Ophthalmol Scand 2000. [Google Scholar] [Crossref]
- R Notivol, D Bertin, D Amin, A Whitling, M Kennedy, P C Cockrum. Comparison of topical tobramycin—dexamethasone with dexamethasone—neomycin—polymyxin and neomycin—polymyxin—gramicidin for control of inflammation after cataract surgery: Results of a multicenter, prospective, three-arm, randomized, double-masked, controlled, parallel-group study. Clin Ther 2004. [Google Scholar] [Crossref]
- F Aptel, C Colin, S Kaderli, C Deloche, A M Bron, M W Stewart. Management of postoperative inflammation after cataract and complex ocular surgeries: a systematic review and Delphi survey. Br J Ophthalmol 2017. [Google Scholar] [Crossref]
- R K. Rajpal, L Roel, R Siou-Mermet, T Erb. Efficacy and safety of loteprednol etabonate 0.5% gel in the treatment of ocular inflammation and pain after cataract surgery. J Cataract Refract Surg 2013. [Google Scholar] [Crossref]
- F C DeCroos, N A Afshari. Perioperative antibiotics and anti-inflammatory agents in cataract surgery. Curr Opin Ophthalmol 2008. [Google Scholar] [Crossref]
- T Tripathi, H Alizadeh. Significance of arachidonic acid in ocular infections and inflammation. Inflamm Cell Signal 2014. [Google Scholar]
- B Gaynes. Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension. Clin Ophthalmol 2008. [Google Scholar] [Crossref]
- C R G Espiritu, M E A Sy, T L G Tayengco. Efficacy and Tolerability of a Combined Moxifloxacin/Dexamethasone Formulation for Topical Prophylaxis in Phacoemulsification: An Open-Label Single-Arm Clinical Trial. J Ophthalmol 2011. [Google Scholar] [Crossref]
- L Apt, S J Isenberg, R Yoshimori, A Spierer. Outpatient Topical Use of Povidone-iodine in Preparing the Eye for Surgery. Ophthalmol 1989. [Google Scholar] [Crossref]
- L L Freitas, E Soriano, C Muccioli, A L Höfling-Lima, R Belfort. Efficacy and tolerability of a combined moxifloxacin/dexamethasone formulation for topical prophylaxis and reduction of inflammation in phacoemulsification: a comparative, double masked clinical trial. Curr Med Res Opin 2007. [Google Scholar] [Crossref]
- P Costello, S J Bakri, P M Beer, R J Singh, N S Falk, G B Peter. Vitreous penetration of topical moxifloxacin and gatifloxacin in humans. Retina 2006. [Google Scholar] [Crossref]
- R Solomon, E D Donnenfeld, H D Perry. Penetration of topically applied gatifloxacin 0.3%, moxifloxacin 0.5%, and ciprofloxacin 0.3% into the aqueous humor. Ophthalmol 2005. [Google Scholar]
- H R Katz, S Masket, S S Lane, K Sall, S C Orr, R D Faulkner. Absorption of Topical Moxifloxacin Ophthalmic Solution Into Human Aqueous Humor. Cornea 2005. [Google Scholar] [Crossref]
- L B Arbisser. Safety of intracameral moxifloxacin for endophthalmitis prophylaxis during cataract surgery. 2007. [Google Scholar]
- C R G Espiritu, V L Caparas, J G Bolinao. Safety of prophylactic intracameral moxifloxacin 0.5% ophthalmic solution in cataract surgery patients. J Cataract Refract Surg 2007. [Google Scholar] [Crossref]
