- Visibility 179 Views
- Downloads 23 Downloads
- DOI 10.18231/j.ijceo.2025.005
-
CrossMark
- Citation
To study the extent of regression of retinal neovascularisation in proliferative diabetic retinopathy following laser photocoagulation of the ischaemic retina
- Author Details:
-
Nadeem
-
Madhura Mukherjee
-
Nazneen Nazm *
Introduction
Diabetes mellitus (DM) is a major medical problem throughout the world and also in our country. Prevalence of diabetes in India is 2.4% in rural population and 4.0-11.6% in urban population.[1] Worldwide, 150 million cases are currently diagnosed with diabetes and it is expected to double by 2025.1 Diabetic mellitus majorly occurs in 2 forms, Type I (Insulin Dependent DM) and Type II (Non Insulin Dependent DM). Diabetes mellitus leads to diabetic retinopathy (DR) after a few years. It is the commonest retinal vascular disorder. Various risk factors which lead to diabetic retinopathy are duration of diabetes, age at diagnosis of diabetes, poor metabolic control, nephropathy, hypertension, smoking, obesity, hyperlipidemia, anemia etc.[2] The basic components of the damaging process are microvascular and macrovascular leakage and capillary closure. Various clinical features of DR are microaneurysms (earliest sign of DR), retinal hemorrhages (dot & blot and flame shaped), hard exudates, cotton wool spots, venous changes (looping, beading), intraretinal microvascular abnormalities (IRMA) in NPDR and retinal neovascularisation in PDR.[3] Diabetic retinopathy (DR) is one of the major causes of legal blindness in adults of working age group worldwide. Diabetic Retinopathy progresses from mild non-proliferative diabetic retinopathy (NPDR) to moderate, severe and very severe NPDR to proliferative diabetic retinopathy (PDR). The estimated 17.6% patients with moderate NPDR and 5.8% patients with mild NPDR progress to PDR within 5 years of diagnosis.[4], [5]
PDR is characterized by the growth of new abnormal vessels on the retina or optic disc and it includes:-
New vessels at the disc (NVD): Neovascularisation on or within one disc diameter of the optic nerve head, New vessels elsewhere (NVE) - neovascularisation further away from the disc and New vessels on the iris (NVI) –rubeosis iridis. PDR is the commonest cause of severe visual loss (vision ≤ 5/200) in people with diabetes as it may lead to vitreous haemorrhage, macular edema, tractional retinal detachment and rhegmatogenous retinal detachment.[6]
Some of the treatment modalities currently used for PDR are as follows: 1) Treatment of diabetes and other systemic risk factors which exacerbate PDR. 2) Panretinal Laser photocoagulation 3) Intravitreal anti VEGF treatment 4) Pars plana vitrectomy
Laser photocoagulation (pan retinal photocoagulation) Laser photocoagulation has been the standard treatment for PDR for several decades after the landmark trial-DRS i.e. Diabetic Retinopathy Study (DRS). PRP causes regression of neovascularisation by decreasing VEGF production from the ischemic retina.[7], [8]
However, PRP has some limitations.
It requires a cooperative patient
It requires a clear view of the retina.
Peripheral vision is permanently lost with the destruction of the lasered peripheral retina
Transient decline in visual acuity (VA) due to increase in macular edema has also been reported.
It is the gold standard for preventing blindness and maintaining baseline visual acuity in cases of Proliferative diabetic retinopathy.[7], [8]
Anti- VEGF agents act by inhibiting the actions of VEGF and hence, decrease the retinal neovascularisation. It is the current standard of care for vision-impairing centre-involving diabetic macular edema.[9], [10] Some recent studies have also shown that anti-VEGF treatment is as good as PRP in PDR but most of the subjects in these studies had PDR without high-risk characteristics.[9] Also, the results of PRP are sustained for a much longer duration (years) whereas the effect of anti- VEGF injections is very short lived (weeks to months) and thus, repeated injections are required. In the initial phase of therapy, these injections have to be given monthly. Thus, the cost of treatment with anti-VEGF injections is much higher than PRP. It has also been seen that when PDR patients do not follow up diligently after their initial anti VEGF or PRP therapy, then the patients who had received anti VEGF therapy fared much worse than the patients who had received PRP even after they restarted following up regularly. They fared much worse in terms of visual acuity, DME, NVD, tractional RD and NVI.5 Therefore PRP remains the standard of care for PDR, particularly if the patients are unlikely to follow up regularly for several years and if the patients find it difficult to afford repeated anti-VEGF injections.
Pars plana vitrectomy Indications for pars plana vitrectomy in diabetic retinopathy include:
Severe persistent vitreous haemorrhage (most common indication)
Progressive tractional RD
Combined tractional and rhegmatogenous RD
Premacular retrohyaloid haemorrhage
It is typically combined with extensive endolaser PRP.
It has been seen that PRP does help in reducing retinal neovascularisation and chances of vitreous haemmorhage and tractional retinal detachment but it has also been seen in some studies that it does not reduce the retinal neovascularisation in many cases and many patients require repeated PRPs and some retinal neovascularisation do not regulates despite multiple PRPs and they develop recurrent vitreous haemorrhage and tractional retinal detachments. Hence, we undertook this study to find out what is the exact extent of regression of neovascularisation with PRP and what are the factors that correlate with this variation in response.
Materials and Methods
Present prospective study was conducted in subjects with Proliferative Diabetic Retinopathy attending the ophthalmology department at ESI-PGIMSR and Model Hospital, Basaidarapur, New Delhi from October 2019- April 2021.
Inclusion criteria
Proliferative diabetic retinopathy.
Age - 18 years and above.
Exclusion criteria
Patients with active intra-ocular inflammation
Moderate or dense vitreous haemorrhage precluding PRP
Significant fibrovascular proliferation
Tractional retinal detachment involving or threatening the macula
The sample size calculated was 71 but as it was a time bound study of 1 and 1/2 years and the subjects were followed for a minimum of 3 months, hence we studied 38 subjects (sample of convenience) on the basis of the following calculations. The study of Mahiul M. et al observed that retinal neovasularisation regression with PRP was seen in 76% of subjects at 12 weeks. Taking this value as reference, the minimum required sample size with 10% margin of error and 5% level of significance was 71 patients. Due to time constraints and long follow up required of the subjects, total sample size taken was calculated to be 36 with margin of error as 14%.
A thorough history was taken from the subject and each subject was thoroughly evaluated prior to therapy. The following examination was performed on each subject:
Ocular examination
Best corrected visual acuity testing for distance and near – It was done on ETDRS charts for English-literate subjects and for subjects who did not know the English alphabets, it was done by using Snellens charts – Hindi, number or Landolt C charts.
Refraction- Refraction was done to find out the best corrected visual acuity and for getting the corrected area of retinal neovascularisation on the fundus photograph on the fundus camera (Zeiss 450 plus).
Keratometry: Keratometry was done for getting the corrected area of retinal neovascularisation on the fundus photograph.
Slit lamp examination including 90D examination: Slit lamp examination was done to evaluate the anterior segment and the posterior segment. NVI was specifically looked for at high magnification. Gonioscopy was done if NVI was observed or the intraocular pressure was high.
Intraocular pressure: IOP was measured by Goldmann’s applanation tonometer.
Indirect ophthalmoscopy: Indirect ophthalmoscopy was done to examine the vitreous and the retina paying particular attention to the areas of neovascularisation, and the amount of fibrous element and any tractional or rhegmatogenous retinal detachment.
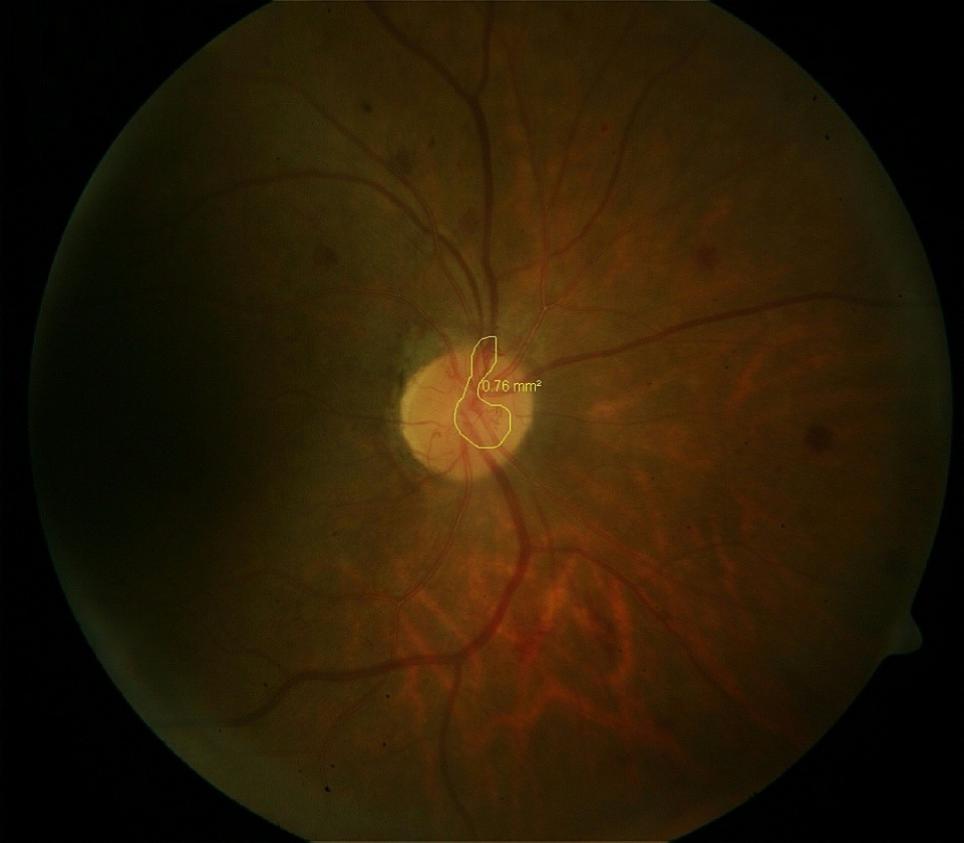
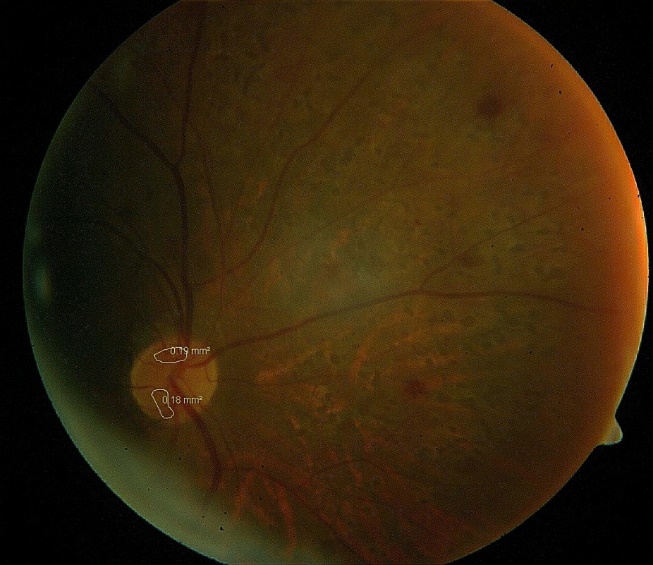
Fundus photograph: Fundus photograph was taken to document the diabetic retinopathy and the areas of retinal and disc neovascularisation. The areas of neovascularisation were measured using the software inbuilt in the fundus camera (Zeiss Fundus Camera 450 plus).
Fundus fluorescein angiography: Fundus fluorecein angiography was done in subjects with normal serum creatinine to document the areas of ischemic retina, retinal neovascularisation and the macular edema.
Various systemic investigations such as blood sugar (fasting and post prandial), serum HbA1c, complete blood count (hemoglobin), serum creatinine, fasting lipid profile were done and the blood pressure measured.
Intervention
The subjects fulfilling the inclusion and exclusion criteria was undergo laser photocoagulation of the ischemic retina using double frequency Nd Yag laser. Laser spots of 500 microns of medium intensity were placed one spot size apart over the ischemic retina. The nasal proximity of the photocoagulation to the disc was not closer than 500 µm and the temporal proximity to the fovea was not closer than 3000 µm from the centre of fovea. The superior and inferior limit was not more posterior than 1 row within the temporal arcades. Laser photocoagulation of the ischemic retina was done in 1 to 3 sessions, with the sessions repeated weekly till the laser photocoagulation was completed. Anti VEGF injection (Ranibizumab 0.05mg in 0.05ml) was given intravitreally if the patient had centre involving macular edema with vision less than or equal to 6/12.
Follow up
Following panretinal photocoagulation, subjects were followed up every 2 weeks and their visual acuity and indirect ophthalmoscopy was repeated. Fundus photograph and measurement of the area of retinal neovascularisation was repeated monthly for 3 months. At the end of 3 months, comparison of the retinal neovascularisation, as measured on the fundus camera at the beginning of the study and at 3 months after retinal photocoagulation was done and the extent of regression noted. The amount of regression was correlated with the different parameters such as age, duration of diabetes, type of diabetes, HbA1c, size of neovascularisation and serum hemoglobin.
Statistical analysis
The recorded data was compiled and entered in a spreadsheet computer program (Microsoft Excel 2019) and then exported to data editor page of SPSS version 15 (SPSS Inc., Chicago, Illinois, USA). Quantitative variables were described as means and standard deviations or median and interquartile range based on their distribution. Qualitative variables were presented as count and percentages. For all tests, confidence level and level of significance were set at 95% and 5% respectively.
Results
This is a prospective study on eyes with PDR. A total of 38 eyes of 22 subjects fulfilled the inclusion and exclusion criteria were included in the study.
|
Age group (years) |
Number of subjects (22) |
Percentage of subjects (%) |
Mean age (years) |
|
<40 |
0 |
0.00% |
0 |
|
40-50 |
8 |
36.36% |
46.07 |
|
50-60 |
10 |
45.45% |
58.87 |
|
>60 |
4 |
10.52% |
65.28 |
Most of our patients were in the age group of 40-50 years and 50-60 years.([Table 1]) In our study population, 16 (72.72%) of the subjects were males with age (mean ± SD) 60.00± 4.20 years were more than females 6 (27.27%) with age (mean ± SD) 51.56± 5.41 years. Maximum number of eyes (71.05%.) having proliferative diabetic retinopathy have HbA1C of range in between 6.6 to 8.5 gm%. Maximum number of eyes 18 (47.37%) have 10-20 years of diabetes duration.
|
VA (Logmar Scale) |
Baseline |
After 3 Months Of PRP |
|
0.00-0.28 |
5(13.16%) |
7(18.42%) |
|
0.30-0.98 |
22(57.89%) |
24(63.16%) |
|
≥1.0 |
11(28.95%) |
7(18.42%) |
|
Total |
38(100%) |
38(100%) |
It has been observed that at baseline, 13% of the eyes had visual acuity of 0.00 to 0.28, 57.89% of the eyes had visual acuity of 0.3 to 0.8 and 28.95% at visual acuity of >=1.0 in logMAR chart. The vision is improved by 18.42% in 0.00-0.28, improved by 63.16% in 0.3- 0.8 score and 18.42% in 0.0-0.2 lines in logMAR chart.([Table 2])
|
VA in 3 months |
Number of Eyes |
Percentage of Eyes (%) |
|
Vision maintained |
3 |
7.89% |
|
Vision improved |
30 |
78.95% |
|
Vision decreased |
5 |
13.16% |
|
Total |
38 |
100% |
In our subjects, visual acuity improved in 30 (78.95%) of the eyes, visual acuity was maintained in 3(7.89%) of the eyes and the visual acuity decreased in 5(13.16%) of the eyes.([Table 3])
The area of neovascularisation in our patients ranged from 0.42 mm2 to 9.23 mm2. Most of our eyes had neovascularisation in the range of 0 to 3 mm2. After 3 months of PRP, all the eyes showed regression of neovascularisation to a significant extent (p=0.003).([Table 4])
|
Variables |
Area of NV (mm2) Mean ± SD |
Regression from Baseline |
p value |
|
|
(mm2) |
% |
|||
|
Baseline |
3.32±2.44 |
- |
- |
|
|
1 month after PRP |
2.72±2.36 |
0.6 |
26.98% |
<0.001 HS |
|
2 months after PRP |
1.91±2.16 |
1.41 |
56.78% |
<0.001 HS |
|
3 months after PRP |
1.5±2.04 |
1.82 |
69.51% |
<0.001 HS |
Mean area of neovascularisation at baseline was 3.32±2.44 mm2 and regressed to 1.5±2.04 mm2 by 3 months, there was regression of 69.51% which is statistically highly significant. It has been observed that no eye regressed completely by 1 month after PRP however 2 eyes (5.26%) regressed completely after 2 months of PRP and 12 eyes (31.58%) completely regressed by 3 months however 26(68.42%) eyes shows partially regressed by 3 months. ([Table 5])
|
|
Area of NVD (mm2) Mean ± SD |
Regression from Baseline |
p value |
|
|
(mm2) |
% |
|||
|
Baseline |
0.28±0.24 |
|
|
|
|
1 month |
0.21±0.21 |
0.07 |
25% |
<0.001 HS |
|
2 months |
0.14±0.180 |
0.14 |
50% |
<0.001 HS |
|
3 months |
0.08±0.04 |
0.20 |
71.4% |
<0.001 HS |
Mean area of NVD at baseline was 0.28±0.24 mm2 and regressed to 0.08±0.04 mm2 by 3 months, there was regression of 71.4% which is statistically highly significant (p<0.001). Complete regression of NVD was seen in 7(25%) PDR eyes and partial regression of NVD was seen in 21(75%) PDR eyes within 3 months after PRP. ([Table 6])
|
NVE Area (mm2) |
Baseline |
3 months |
|
0.0-3.0 |
22 |
31 |
|
3.1-6.0 |
11 |
5 |
|
6.1-9.0 |
5 |
2 |
The area of NVE in our patients at baseline ranged from 0.21 mm2 to 8.61 mm2. Most of our patients had NVEs in the range of 0.21 to 3.0 mm2 as can be seen from the [Table 7]. After 3 months of PRP all the NVEs showed regression to a significant extent (p=0.006). Complete regression of NVE was seen in 13(34.21%) of PDR eyes and partial regression was seen in 25(65.79%) of PDR eyes within 3 months after PRP.
|
|
Amount of regression of neovascularisation |
|||
|
HbA1c (gm%) |
≤ 50% |
50-75% |
>75% |
Total |
|
≤6.5 |
0 |
2(50%) |
2(50%) |
4 |
|
6.6-8.5 |
12(44.4%) |
11(40.7%) |
4(14.8%) |
27 |
|
8.6-10.5 |
3(42.9%) |
4(57.1%) |
0 |
7 |
In patients with HbA1c of 6.6-8.5 gm%, 4(14.8%) of the 27 eyes had regression of more than 75%, 11(40.7%) had regression of 50-75% and 12(44.4%) had regression of less than 50% whereas in patients with HbA1c of 8.6-10.5 gm%, none of the 7 eyes had regression of more than 75%, 4(57.1%) had regression of 50-75% and 3(42.9%) had regression of less than 50% whereas in patients with HbA1c of less than 6.5gm%, 2(50%) of the 4 eyes had regression of more than 75%, 2(50%) had regression of 50-75% and none had regression of less than 50%.
In patients with DM duration of 10-20 years, 7(38.9%) of the 18 eyes had regression of more than 75%, 6(33.3%) had regression of 50-75% and 5(27.8%) had regression of less than 50% whereas in patients with DM duration of more than 20 years, 1(12.5%) of the 8 eyes had regression of more than 75%, 1(12.5%) had regression of 50-75% and 6(75%) had regression of less than 50% whereas in patients with DM duration of less than 10 years, 3(25%) of the 12 eyes had regression of more than 75%, 5(41.7%) had regression of 50-75% and 4(33.3%) of the 12 eyes had regression of less than 50%.
Discussion
Present study reported the amount of regression of retinal neovascularisation in PDR following laser photocoagulation (PRP) of the ischaemic retina and the factors associated with the amount of regression. In our country, it is not very economic and practical for patients to come for repeated monthly evaluations and receive multiple intravitreal anti VEGF injections and thus PRP remains a very useful modality for treating PDR as it gives much more long-lasting benefits as compared to intravitreal injections and patients have to make a lesser number of hospital visits. Hence, we undertook this study to study the amount of regression of retinal neovascularisation in PDR following laser photocoagulation (PRP) of the ischaemic retina in our study population and the factors associated with the amount of regression.
In our study of 38 eyes of 22 patients, 73% were males and 27% were females. Out of the 22 patients, there were 8 cases in the 40-50 age group, 10 cases in the 50-60 age group and 4 were above > 60 years. Also there were 22.72% of cases with type 1 DM and 77.27% with type 2 DM.
In our study the area of neovascularization (NVD+NVE) regressed by 69.51% after 3 months of PRP. There was a regression of 71.4% of NVD and 51.05% of NVE in 38 eyes. Feng HH et al[11] observed that in their subjects, the baseline mean area of neovascularisation elsewhere (NVE) was 1.448 ± 1.101mm2 and it regressed to 1.121 ± 0.790 mm2 by 3 months.
In our study of 38 eyes with PDR, all patients (100%) showed some regression of their neovascularisation at 3 months and 26 (68%) showed partial regression and 12 (32%) showed complete regression of their neovascularisation. In contrast Vander et al[12] concluded that 35 eyes (59.3%) had regression of high-risk retinopathy characteristics within 3 months of treatment.
In our study, of the 28 eyes with NVD, 7 eyes (25%) showed complete regression of NVD and 21 eyes (75%) showed only partial regression of NVD after 3 months of PRP. Munira Y et al[13] observed that 3 months after completion of PRP, 20 patients (45.5%) of PDR had complete regression of NV while 24 patients (54.5%) experienced incomplete regression whereas in our study, 12(31.6%) of 38 eyes had complete regression of NV while 26(68.42%) of the 38 eyes showed partial regression.
In our study of 38 eyes with PDR, 30 eyes (79%) of the eyes showed an improvement in their visual acuity due to decrease in their central macular edema due to decreased production of VEGF from the peripheral ischaemic retina after PRP. 3 eyes (8%) maintained their visual acuity while 5 eyes (13%) had a decrease in their visual acuity due to an increase in the central macular edema immediately following PRP. In contrast, Sujatha R. et al[14] observed that after PRP, 74.3% subjects retained their baseline vision but decreased vision was noted in 22% of subjects.
In our study, we observed that the NVD which was present in 28 eyes at baseline regressed to some extent in 100% of the eyes and completely regressed in 7 (25%) eyes at 3 months follow up. Similarly, NVE which was present in 38 eyes at baseline regressed to some extent in 100% of the eyes and completely regressed in 13(34.21%) eyes at 3 months follow up. Mean area of NVD regressed by 71% and mean area of NVE regressed by 51% at 3 months follow up. Kaiser RS et al[15] observed that the NVD which was present in 184 PDR eyes at baseline regressed in 115 eyes at 1 year follow up. Similarly, NVE which was present in 200 eyes at baseline regressed in 146 eyes at 1 year follow up. Hence, NVD showed regression in 62% of the eyes and NVE showed regression in 73% of eyes at 1 year follow up in their study.
In our study, maximum number of eyes 18 (47.37%) had 10-20 years of diabetes duration. We also observed that shorter diabetic duration patients had more medical neglect and many patients were not sure of the actual duration of diabetes. Thus in our study, we observed that PRP is quite effective in treating PDR as it causes significant reduction in the neovascularisation and thus decreases the chances of severe visual loss due to vitreous hemmorhage, tractional retinal detachment, persistent macular edema and combined mechanism (tractional and rhegmatogenous) retinal detachment.
The study would be strengthened if a larger number of subjects could have been included in the study and if we could have had a longer follow up of the subjects. Hence, additional studies with larger datasets are needed to reinforce our results. The study could have been strengthened further if we could have combined treatment with intravitreal anti VEGF injections with PRP for better neovascularisation regression.
Conclusion
In our study, PRP was shown to induce regression of neovascularisation and arrest of progression of diabetic retinopathy. PRP in PDR reduced the risk of severe visual loss. Timely applied laser photocoagulation of the ischaemic retina was effective in causing regression of the already developed neovascularisation and in preventing further development of neovascularisation. PRP is quite effective in causing regression of the abnormal neovascularisation which develops in PDR and which may lead to severe visual loss by causing repeated vitreous hemmorrhage, tractional retinal detachment and rhegmatogenous retinal detachment. But it alone may not suffice for total regression of neovascularisation in all PDR patients and we may need to supplement it with anti VEGF agents. Systemic control of diabetes and the other systemic risk factors are also very important in slowing the progression of the disease.
Source of Funding
There was no financial support concerning this work.
Conflict of Interest
No! Conflict of interest is found elsewhere considering this work.
References
- V Mohan, R Pradeepa. Epidemilogy of diabetes in different Regions of India. Health Administrator 2009. [Google Scholar]
- M Kropp, O Golubnitschaja, A Mazurakova, L Koklesova, N Sargheini, TTKS Vo. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications—risks and mitigation. EPMA J 2023. [Google Scholar]
- AA Alghadyan. Diabetic retinopathy – An update. Saudi J Ophthalmol 2011. [Google Scholar]
- JW Yau, SL Rogers, R Kawasaki, EL Lamoureux, JW Kowalski, T Bek. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012. [Google Scholar]
- A Moshfeghi, V Garmo, D Sheinson, A Ghanekar, I Abbass. Five-year patterns of diabetic retinopathy progression in us clinical practice. Clin Ophthalmol 2020. [Google Scholar]
- DA Antonetti, R Klein, TW Gardner. Diabetic retinopathy. N Engl J Med 2012. [Google Scholar]
- S Sivaprasad, AT Prevost, JC Vasconcelos, A Riddell, C Murphy, J Kelly. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 2017. [Google Scholar]
- . Preliminary report on effects of photocoagulation therapy. The Diabetic Retinopathy Study Research Group. Am J Ophthalmol 1976. [Google Scholar]
- A Arrigo, E Aragona, F Bandello. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann Med 2022. [Google Scholar]
- SB Bressler, WT Beaulieu, AR Glassman, JG Gross, M Melia, E Chen. Panretinal Photocoagulation Versus Ranibizumab for Proliferative Diabetic Retinopathy: Factors Associated with Vision and Edema Outcomes. Ophthalmology 2018. [Google Scholar]
- HE Feng, YU Weihong, F Dong. Observation of retinal neovascularization using optical coherence tomography angiography after panretinal photocoagulation for proliferative diabetic retinopathy. BMC Ophthalmol 2021. [Google Scholar]
- JF Vander, JS Duker, WE Benson, GC Brown, JA Mcnamara, RB Rosenstein. Long-term stability and visual outcome after favorable initial response of proliferative diabetic retinopathy to panretinal photocoagulation. Ophthalmology 1991. [Google Scholar]
- Y Munira, E Zunaina, Z Sakinah. Effect of panretinal photocoagulation on retinal nerve fiber layer thickness and vision-related quality of life in proliferative diabetic retinopathy patients. J Biomed Clin Sci 2021. [Google Scholar]
- R Sujatha, S Samreen. Evaluation of Visual Outcome in Proliferative Diabetic Retinopathy after Panretinal Photocoagulation. J Evol Med Dent Sci 2019. [Google Scholar]
- RS Kaiser, MG Maguire, JE Grunwald, D Lieb, B Jani, AJ Brucker. One-year outcomes of panretinal photocoagulation in proliferative diabetic retinopathy. Am J Ophthalmol 2000. [Google Scholar]
How to Cite This Article
Vancouver
Nadeem , Mukherjee M, Nazm N. To study the extent of regression of retinal neovascularisation in proliferative diabetic retinopathy following laser photocoagulation of the ischaemic retina [Internet]. Indian J Clin Exp Ophthalmol. 2025 [cited 2025 Sep 04];11(1):24-30. Available from: https://doi.org/10.18231/j.ijceo.2025.005
APA
Nadeem, , Mukherjee, M., Nazm, N. (2025). To study the extent of regression of retinal neovascularisation in proliferative diabetic retinopathy following laser photocoagulation of the ischaemic retina. Indian J Clin Exp Ophthalmol, 11(1), 24-30. https://doi.org/10.18231/j.ijceo.2025.005
MLA
Nadeem, , Mukherjee, Madhura, Nazm, Nazneen. "To study the extent of regression of retinal neovascularisation in proliferative diabetic retinopathy following laser photocoagulation of the ischaemic retina." Indian J Clin Exp Ophthalmol, vol. 11, no. 1, 2025, pp. 24-30. https://doi.org/10.18231/j.ijceo.2025.005
Chicago
Nadeem, , Mukherjee, M., Nazm, N.. "To study the extent of regression of retinal neovascularisation in proliferative diabetic retinopathy following laser photocoagulation of the ischaemic retina." Indian J Clin Exp Ophthalmol 11, no. 1 (2025): 24-30. https://doi.org/10.18231/j.ijceo.2025.005
