- Visibility 497 Views
- Downloads 64 Downloads
- DOI 10.18231/j.ijceo.2024.094
-
CrossMark
- Citation
Ethambutol induced optic neuropathy: A remerging ocular emergency needing strict vigilance and screening
- Author Details:
-
Monika Dahiya *
-
Manisha Rathi
-
Mohit Dua
-
Sumit Sachdeva
-
Ruchi Dabas
Introduction
In 2019, India notified 24 lakh tuberculosis patients with estimated incidence of 199 patients per 100,000 population accounting for almost 26% of global incidence, making India a TB capital.[1], [2] With aim of elimination of TB by 2025, revised national tuberculosis control program (RNTCP) was revised in 2016 with daily ATT regimen and ethambutol was made a part of both the intensive as well as continuation phase of treatment, therefore increasing cumulative dose of ethambutol, making patients more prone for developing ethambutol induced optic neuropathy.[3]
Ethambutol is a first line drug used in ATT which is considered safe and have least systemic side effects, but it also has potential ocular and hepatic side effects. EON is dose and duration dependent toxicity, therefore the reported incidence of EON widely varies, ranging from 1-18% in various studies.[4], [5], [6], [7], [8] Initially, ethambutol induced ocular toxicity was considered to be reversible, but over the time, it is proven to be partially reversible in initial stage and irreversible in late stage.[9], [10], [11] Therefore, early detection and timely management is the only way to save sight from EON.
Ethambutol toxicity typically presents between 3-5 months but it can present as early as 1.5 months and as late as 12 months of starting the therapy.[12], [13] EON usually present as bilateral subacute, bilateral, painless loss of central vision. Dyschromatopsia or fading of colours is the earliest sign of toxicity and usually seen in red-green spectrum. [14], [15] In 13-50% cases, patients are asymptomatic but have subclinical toxicity of ethambutol as assessed on visual evoked potential (VEP) and optical coherence tomography (OCT). [16], [17] The exact mechanism of ethambutol toxicity is still not known; however it is hypothesized that ethambutol acts as chelating agent which disrupts one of the several metal- containing enzyme systems.[18] Ethambutol induced optic neuropathy was described by Leibold into two types: i.e., axial neuritis and periaxial neuritis. Axial neuritis is the most common types which involves the papillomacular fibres of visual pathway, resulting in visual loss, colour vision impairment and caecocentral scotomas. In periaxial neuritis, peripheral defects are noted, but visual acuity remain unaffected.[19]
In EON cases, fundus examination may be normal in initial stages, hyperaemic disc, disc edema, disc pallor and optic atrophy in later stage. The most common visual field defect on perimetry is caecocentral scotomas, but bitemporal defects and peripheral field constriction have also been reported.[3] In subclinical toxicity diagnosis, contrast sensitivity measurement, VEP and OCT is quite effective.[9] On OCT, changes in form of RNFL and GCL thinning is reported in symptomatic as well as asymptomatic patients. On VEP, increase in P100 latency is noticed, both in asymptomatic as well as symptomatic cases. However, it is still not clear how many of asymptomatic cases may go on to develop clinical EON.
Till date, there is no effective treatment for EON. Early detection and stopping ethambutol is the only remedy to prevent further visual loss. The damage is reversible only in early stage and due to revised RNTCP 2016, cumulative dose of ethambutol is increased drastically, making patients more at risk for EON. Ethambutol toxicity can significantly hamper patient’s quality of life due to visual impairment, loss of contrast sensitivity and colour vision impairment. In current scenario, diagnosing EON at an early stage and discontinuing ethambutol is the need of the hour. With this background, this study was conducted to investigate the clinical manifestations and incidence of ethambutol induced optic neuropathy (EON) and identify its risk factors.
Materials and Methods
A cross-sectional, hospital based observational study was conducted in 100 patients, who were on ATT and refereed for ophthalmic examination for diminution of vision from CTB department of our institute. This study was done after taking clearance from Institutional Ethics Committee and patients were included after taking their informed written consent. The following data was collected in every patient regarding age, gender, co-morbid conditions, clinical manifestation of TB, duration and dose of ethambutol. Complete ophthalmic examination was conducted in every patient including BCVA, color vision, RAPD, fundus examination, visual field analysis and OCT RNFL & GCL. Statistical analysis of the data was done and represented suitably using appropriate statistical tests that included Chi Square test for checking associations and T test for comparing quantitative data with the help of SPSS ver. 26.0 software. All p-values reported were two-sided and a value less than 0.05 was considered significant.
Results
Out of 100 patients refereed from CTB department for ophthalmic evaluation, only 18 patients were diagnosed with EON, out of which 12 were male and 6 were females with M:F ratio of 2:1. The patients were referred to ophthalmology department once they developed visual complaints and no ophthalmic evaluation was done before starting ATT. The age ranged from 17-75 years (mean: 46+12.42 years) ([Figure 1]). In this study, incidence of EON was 1.8%.

The average dose of ethambutol was 800 mg/day. The duration of treatment with ethambutol ranged from 1-5 month when they were referred to our department for diminution of vision. The most common type of TB was pulmonary TB (61%) followed by spinal (28%) and genital TB (11%) ([Figure 2]).

The most common presenting feature of EON was bilateral diminution of vision (89%) followed by abnormal color vision (11%). Color vision testing was done in all cases of EON by Ishihara chart and out of 18 cases, 14 patients were having defective color vision in red-green spectrum. On examination, RAPD was seen in 40% cases because of asymmetrical optic nerve involvement. Fun and disc abnormality was present in 66.66% (12/18) cases. On dilated fundus examination, 6 patients were having normal disc, while 3 were having hyperemic disc, 4 were having temporal pallor while 5 were having complete disc pallor ([Figure 3]).
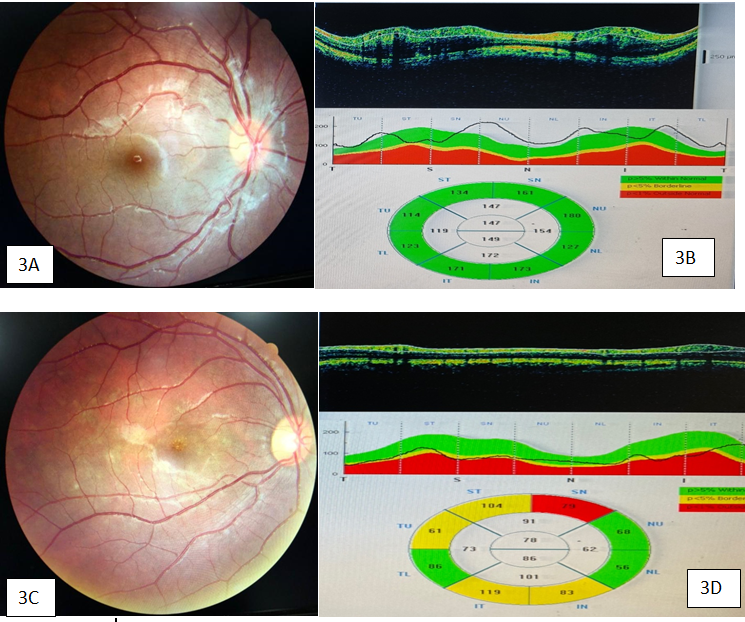
On HFA, visual field abnormality was seen in 50% cases (9/18) only. The most common visual field abnormality was central/caecocentral scotoma in 89% cases (8/9), while in 1 patient, bitemporal visual field defects. OCT changes were seen in 90% cases, showing RNFL and GCL changes. Out of 18 cases, 6 patients were having normal RNFL and GCL parameters, 3 patients were having increase in RNFL thickness and GCL thinning and 9 patients were having RNFL and GCL thinning. In this study, majority of patients (50%) were having RNFL and GCL thinning. RNFL and GCL changes depend on stage of EON and disc appearance. In early stages, RNFL thickness may be normal or even increased if disc become hyperemic, however as the disease progress, RNFL thinning will eventually occur. The duration and dose of ethambutol medication, advanced age (>65 years), HTN, chronic smoking and presence of renal dysfunction were shown to be positively correlated with EON development but statistical correlation could not be found, because of small sample size.
Discussion
The overall reported incidence of ethambutol-induced optic neuropathy in patients receiving ethambutol in ATT regimen is around 1% which is dose and duration dependent.[20] In clinical practice, there is no safe dose for ethambutol as ethambutol related toxicity is reported at a dose as low as 12.3 mg/kg/day.[21] The incidence of ethambutol‐related ocular toxicity is largely dose dependent, it widely varies from 1%–2.5% at a dose of 15 mg/kg per day, 5%–6% at a dose of 25 mg/kg/day and almost 18% at 35 mg/kg/day dose.[22], [8], [9], [10], [11], [12] As per new guidelines, average dose of ethambutol is 20mg/kg/day in adults while in children, it is 25mg/kg/day which is significantly high. The risk of EON increases in conditions like malnutrition, advanced age, alcohol, tobacco, renal dysfunction, diabetes and hypertension. The risk increases even more in concurrent use of isoniazid and linezolid, as they are also neurotoxic.[8], [9]
It is still a matter of debate whether EON is reversible or not. The reported recovery rate of ethambutol induced ocular toxicity was around 50% in many studies.[21], [23] However, patients who recover from EON, normal visual functions are not regained completely, as visual field, colour vision and contrast sensitivity defects persist. Till date, there is no definite cure for ethambutol induced ocular toxicity. Therefore, early diagnosis and timely intervention is the key to its management. Ethambutol should be stopped immediately with supplementation of zinc, copper and vitamin; specially methylcobalamine and pyridoxine to prevent further deterioration of visual functions.
The course of EON is unpredictable and there is no specific treatment, so primary prevention is the best way to combat this disease. Therefore, it is must to make patients as well as health care workers, aware of this potential side effect of drug. The information material regarding EON and its impact on vision as well as quality of life, should be provided to patients as well as health care providers in the form of education pamphlets. In ideal scenario, each patient should undergo baseline ophthalmic examination including visual acuity, colour vision, fundus examination and visual fields before starting ATT followed by regular follow up. However, because of excessive TB burden in our country, it is not practically feasible to perform all these tests in every patient who is on ethambutol, therefore all patients with high risk factors must undergo baseline ophthalmic evaluation.
Patients and their caregivers should be educated about potential visual disturbances and to seek immediate health care in such situation. Patients with high risk factors for EON should be provided an Amsler’s grid or pocket Snellen chart for home monitoring. At community level, all health care providers, especially the field workers like Accredited Social Health Activist (ASHA) worker or Multipurpose Health Worker (MPWs) must be educated about potential side effects of ethambutol and they must be trained to identify high risk patients for EON. The treating physician should note any pre- existing visual impairment and refer to ophthalmologist for detailed workup. If any visual dysfunction occurs on follow-up, it should be notified to either RNTCP or Pharmacovigilance Programme of India (PvPI). It will help in calculating the incidence of EON and also identify its high-risk factors.
Conclusion
We strongly recommend each patient should undergo baseline ophthalmic evaluation before commencement of ATT followed by regular evaluation of VA, color vision and visual fields. If not practically possible, at least patients with high risk factors should be identified by treating physician and referred to an Ophthalmologist for all baseline workup to prevent EON.
Source of Funding
None.
Conflict of Interest
None.
References
- . India TB report 2020. Central TB Division, Ministry of Health and Family Welfare, India 2020. [Google Scholar]
- . Global tuberculosis report 2019. 2019. [Google Scholar]
- . India TB report 2017. Central TB Division Directorate General of Health Services, Ministry of Health and Family Welfare 2017. [Google Scholar]
- JL Goyal, D Sarmi, NP Singh, A Bhatia. Evaluation of visual functions in patients on ethambutol therapy for tuberculosis: A prospective study. J Commun Dis 2003. [Google Scholar]
- U Kim, JM Hwang. Early stage ethambutol optic neuropathy: Retinal nerve fiber layer and optical coherence tomography. Eur J Ophthalmol 2009. [Google Scholar]
- SJ Chai, R Foroozan. Decreased retinal nerve fibre layer thickness detected by optical coherence tomography in patients with ethambutol-induced optic neuropathy. Br J Ophthalmol 2007. [Google Scholar]
- CI Zoumalan, M Agarwal, AA Sadun. Optical coherence tomography can measure axonal loss in patients with ethambutol induced optic neuropathy. Graefes Arch Clin Exp Ophthalmol 2005. [Google Scholar]
- FW Fraunfelder, AA Sadun, T Wood. Update on ethambutol optic neuropathy. Expert Opin Drug Saf 2006. [Google Scholar]
- RE Carr, P Henkind. Ocular manifestations of ethambutol. Arch Ophthalmol 1962. [Google Scholar]
- I Harada. Two cases of neuritis retrobulbaris due to orally administered ethambutol. Nihon Ganka Kiyo 1963. [Google Scholar]
- A Grzybowski, M Zulsdorff, H Wilhelm, F Tonagel. Toxic optic neuropathies: An updated review. Acta Ophthalmol 2015. [Google Scholar]
- A Melamud, GS Kosmorsky, MS Lee. Ocular ethambutol toxicity. Mayo Clin Proc 2003. [Google Scholar]
- P Sivakumaran, AC Harrison, J Marschner, P Martin. Ocular toxicity from ethambutol: a review of 4 cases and recommended precautions. NZ Med J 1998. [Google Scholar]
- N Ezer, A Benedetti, ZM Darvish, D Menzies. Incidence of ethambutol related visual impairment during treatment of active tuberculosis. Int J Tuberc Lung Dis 2013. [Google Scholar]
- BC Polak, M Leys, GH Vanlith. Blue-yellow colour vision changes as early symptoms of ethambutol oculotoxicity. Ophthalmologica 1985. [Google Scholar]
- KW Jin, JY Lee, S Rhiu, DG Choi. Longitudinal evaluation of visual function and structure for detection of subclinical Ethambutol-induced optic neuropathy. PLoS One 2019. [Google Scholar]
- KL Kim, SP Park. Visual function test for early detection of ethambutol induced ocular toxicity at the subclinical level. Cutan Ocul Toxicol 2016. [Google Scholar]
- LM Kahana. Toxic ocular effects of ethambutol. Can Med Assoc J 1987. [Google Scholar]
- JE Leibold. The ocular toxicity of ethambutol and its relation to dose. Ann N Y Acad Sci 1966. [Google Scholar]
- SC Chen, MC Lin, SJ Sheu. Incidence and prognostic factor of ethambutol-related optic neuropathy: 10-year experience in southern Taiwan. Kaohsiung J Med Sci 2015. [Google Scholar]
- SY Choi, JM Hwang. Optic neuropathy associated with ethambutol in Koreans. Korean J Ophthalmol 1997. [Google Scholar]
- JE Leibold. The ocular toxicity of ethambutol and its relation to dose. Ann N Y Acad Sci 1966. [Google Scholar]
- RK Tsai, YH Lee. Reversibility of ethambutol optic neuropathy. J Ocul Pharmacol Ther 1997. [Google Scholar]
How to Cite This Article
Vancouver
Dahiya M, Rathi M, Dua M, Sachdeva S, Dabas R. Ethambutol induced optic neuropathy: A remerging ocular emergency needing strict vigilance and screening [Internet]. Indian J Clin Exp Ophthalmol. 2025 [cited 2025 Sep 08];10(3):545-548. Available from: https://doi.org/10.18231/j.ijceo.2024.094
APA
Dahiya, M., Rathi, M., Dua, M., Sachdeva, S., Dabas, R. (2025). Ethambutol induced optic neuropathy: A remerging ocular emergency needing strict vigilance and screening. Indian J Clin Exp Ophthalmol, 10(3), 545-548. https://doi.org/10.18231/j.ijceo.2024.094
MLA
Dahiya, Monika, Rathi, Manisha, Dua, Mohit, Sachdeva, Sumit, Dabas, Ruchi. "Ethambutol induced optic neuropathy: A remerging ocular emergency needing strict vigilance and screening." Indian J Clin Exp Ophthalmol, vol. 10, no. 3, 2025, pp. 545-548. https://doi.org/10.18231/j.ijceo.2024.094
Chicago
Dahiya, M., Rathi, M., Dua, M., Sachdeva, S., Dabas, R.. "Ethambutol induced optic neuropathy: A remerging ocular emergency needing strict vigilance and screening." Indian J Clin Exp Ophthalmol 10, no. 3 (2025): 545-548. https://doi.org/10.18231/j.ijceo.2024.094
