Introduction
Corneal perforation or impending perforation is a serious eye emergency that requires immediate attention to prevent vision loss and other complications such as adherent leucoma, cataract, secondary glaucoma, choroidal haemorrhage, and endophthalmitis.1 There are several conditions which can predispose corneal perforation, including trauma, infection, autoimmune disease, and various corneal degenerations and dystrophies.2 While the diagnosis of corneal perforation is usually evident with prolapse of intraocular content, in cases of occult perforation or micro-perforation, Seidel's test can be useful to confirm the diagnosis.3 The preoperative medical management for corneal perforation includes the use of preservative-free topical drugs, intravenous antimicrobial agents, oral doxycycline and high doses of vitamin C.4 If there is any suspicion of autoimmune association, a complete systemic workup is mandatory.5 Depending on size, site and aetiology, various surgical techniques such as BCL with or without tissue adhesive (TABCL), Tenon's patch grafting (TPG), corneal full-thickness/lamellar patch grafting (CPG) and therapeutic/tectonic penetrating keratoplasty have been described in the literature to deal with corneal perforation/descemetocele.1, 2, 3, 4, 5, 6
The use of Tenon's patch graft (TPG) for perforation/descemetocele is gaining popularity due to its numerous benefits.7 Firstly it is autologous tissue, so there is no risk of immune rejection. Additionally, the anterior thick part of the Tenon's capsule, which is used for the patch grafting, produces autologous fibroblasts and connective tissue that reduces inflammation, so aids in wound healing and tissue remodelling.8, 9, 10 Most importantly, when donor corneal tissue is unavailable, this procedure can be performed by any anterior segment surgeon without the need for special instruments or HOTA certification, potentially saving many eyes in emergency situations.
In 1998, TPG was introduced as a treatment for leaking blebs after glaucoma filtering surgeries.11 Vajpayee et al. presented their innovative work on Tenon’s patch grafting for repairing corneal perforation at the annual meeting of the American Academy of Ophthalmology in 2012.12, 13 Since then, several case series and reports have been published demonstrating its effectiveness in corneal perforation/descemetocele of varying sizes.12, 13, 14, 15, 16 Considering the successful use of TPG, we conducted a study to evaluate its success in managing corneal perforation/descemetocele of 2-5.5mm in size. This prospective interventional study was conducted in the department of Ophthalmology, S. N. Medical College, U.P. between March 2022 and May 2024. The main goal of the study was to evaluate the success of TPG in restoring and maintaining globe integrity during follow-up. The secondary objectives were to assess visual rehabilitation, any post-operative complications, and the thickness of the cornea (evaluated by ASOCT) in the graft area.
Materials and Methods
A total of 43 eyes with corneal perforations/descemetoceles belonging to 42 patients, who were admitted to the Ophthalmology department of S.N. Medical College, Agra, India, between March 2022 and May 2023, were included in this study. The inclusion criteria required that the patient had a corneal perforation or descemetocele of size between 2-5.5mm, with a visual acuity of at least PL positive and PR accurate. On the basis of inclusion criteria, 3 patients with PL denied eyes were excluded from the study. The remaining 40 patients were treated surgically with tenon's patch grafting, as an emergency procedure, by a single surgeon during this period. The study was approved by the ethical committee of the institute (SNMC/IEC/2022/36), and informed and trial is also registered with CTRI (2024/02/062281).
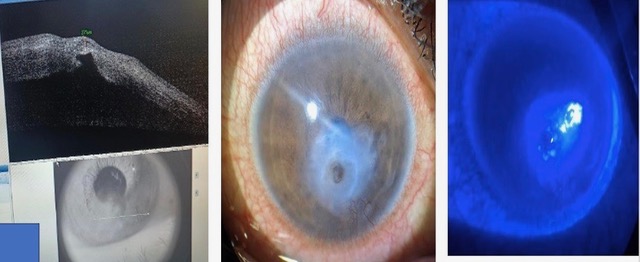
Written consent was obtained from all the patients. In all cases, a detailed history was taken to identify any underlying pathology, visual acuity was assessed, and a slit lamp examination and Schirmer's test were performed. In cases of descemetocele, the thickness in the area of thinning was measured using AS-OCT (Figure 1). Patients' medical history was examined to identify systemic co-morbidities, and depending on the history, they were further investigated through pathological tests such as HbA1c, RA factor, Mantoux test, chest X-Ray, thyroid profile, Hep B, C & HIV to manage the case as a whole. Complete systemic antimicrobial coverage was given to all cases of corneal perforation along with specific topical fortified antimicrobials. To promote corneal healing, patients were administered oral high doses of vitamin C (500mg BD) for a month and Doxycycline (100mg BD) for 21 days in all cases. In addition to the above-mentioned treatment, patients with descemetocele were also given oral acetazolamide (250 mg BD) and topical anti-glaucoma medications. All patients were operated on within 24 hours of presentation under local anaesthesia.
Steps of surgery
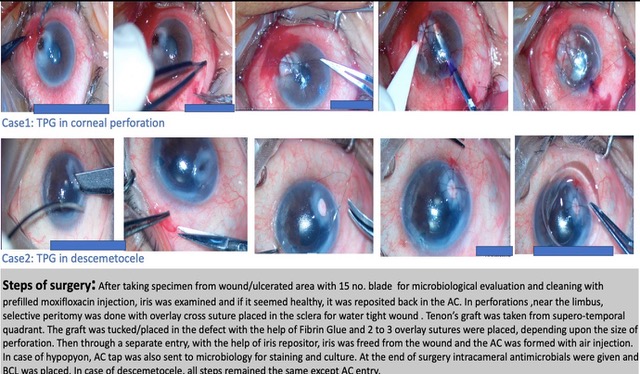
Surgical steps are shown in Figure 2. After placing speculum, the scraping from the wound or ulcerated area along with pseudo-membranes over the prolapsed iris, were sent for microbiological evaluation. Then wound was thoroughly cleaned with preservative free prefilled moxifloxacin injection. Then iris was examined and if the iris appeared healthy, it was reposited otherwise it was excised. In cases where the perforation was close to the limbus, selective peritomy was done to place one bite of an overlay cross suture in the sclera. We took a Tenon's graft of the desired size from the supero-temporal quadrant, three millimetres away from the limbus. After washing the graft thoroughly with BSS, we placed it in the defect and secured it using fibrin glue by applying fibrin glue over the graft and along the sides. As Tenon's is a loose and fluffy tissue, so depending on the size of the defect, we placed two to three overlay sutures with 10-0 nylon over the graft, to ensure a perfectly watertight wound. After making a separate entry, dispersive viscoelastic was injected to the eye. An iris repositor was then used to free the iris from the wound. Afterwards, the anterior chamber (AC) was washed and air was injected to form the AC. If there was hypopyon, AC tap was sent to microbiology for staining and culture sensitivity. At the end of the surgery, intracameral antimicrobial medication was given and a bandage contact lens (BCL) was placed. In case of a descemetocele, all steps were the same except for the entry into the AC.
Good anatomical outcomes following surgery were defined as having a well-formed anterior chamber with normal intraocular pressure (digitally) on the first day post-op. Anatomical failure was considered if the anterior chamber was found to be flat or shallow with a soft eye and seidel's test was used to check for graft dehiscence in such cases. If any issue was found, immediate post-operative interventions, such as additional AMT, cyanoacrylate glue, repeat TPG or a keratoplsty was performed.
Post-operative care and follow up regimen
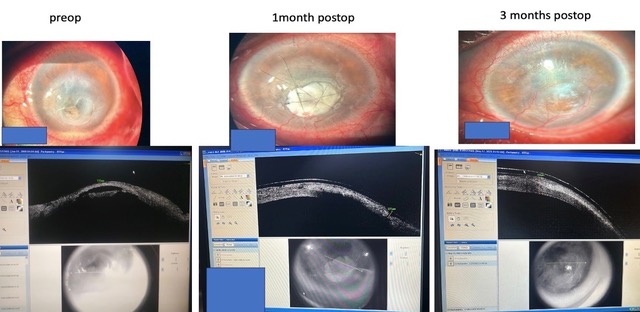
After the surgical procedure, the patient was given systemic antimicrobials, anti-inflammatory drugs, and acetazolamide as necessary. Specific fortified topical antimicrobials were administered hourly for a week, followed by tapering doses over six weeks. Eye drop loteprednol etabonate 0.5% was used four times daily in non-infectious cases from day one post op and in infective cases once the infection was well-controlled and tapered accordingly. Topical Sodium hyaluronate 0.1% six times daily, atropine 1% BD and anti-glaucoma medications were administered also started from day one post-op and continued for a period as and when required. Systemic illnesses were also addressed simultaneously if present. Sutures were removed six weeks after the operation or earlier if they become loose. Patients were reviewed weekly for the first month, then bi-weekly for the next month, and monthly thereafter postoperatively. During follow-up visits, a complete Slit lamp examination and AS-OCT were performed to note characteristic changes in the graft over time (Figure 3).
Results
Out of 40 patients, five patients were lost to follow up so they were excluded from the study analysis. Table 1 provides the demographic information of all the 35 patients. The average age of our study group was 53±10 years. There was no preference in terms of sex or laterality among the participants. Out of 35, corneal perforations were 25 (71.4%) and descemetoceles 10 (28.5%). The TPG was most commonly performed for paracentral followed by peripheral perforation/descemetocele. The mean size of the corneal lesions for corneal perforation was (3.1±1.04 mm), and for descemetocele was (2.45±0.42 mm).
Table 1
Baseline (Preoperative) details of patients (n=35)
Table 2
Summary of the findings of various studies on TPG
|
Article |
Year |
Number of eyes |
Indication |
Size of perforation |
Technique employed |
Outcome |
Mean duration of follow up |
|
Korah et al17 |
2016 |
28 |
Trauma, Sterile perforation, Perforated microbial keratitis (10) |
3-6 mm |
TPG+TA+ BCL |
Adherent leucoma-26, LTFU-1, Evisceration-1 |
4 months |
|
Sharma et al18 |
2019 |
31 |
Trauma (10), neurotrophic keratitis (11), PUK (10 |
3-5mm |
TPG (tuck-in)+TA+BCL |
CS+FAC 27, adherent leucoma 1, Flat AC-3 |
4 months |
|
Chaudhary et al19 |
2021 |
1 |
HZO keratitis |
3x2mm |
TPG+AMG |
CS+FAC |
32 months |
|
Mansour et al20 |
2021 |
1 |
Post-phaco burn |
2.6 mm |
TPG (sutured) |
Well apposed wound |
6 months |
|
Bafna et al21 |
2022 |
22 |
Dry eye (10), neurotrophic keratitis (6), trauma (2), chemical injury (2) and exposure (2) |
3-5 mm |
TPG with fibrin glue |
Graft pseudoectasia (1), graft melting (1) |
1 year |
|
Kushmesh et al22 |
2021 |
85 |
Infectious keratitis (41) autoimmune disorders (30) |
3-6 mm |
TPG PKP |
Graft dehiscence(8), graft displacement(1) |
18 months |
|
Shankar et al23 |
2017-18 |
24 |
Inf keratitis (16) CI/TCI (4) Trauma (1) DED (1) SJS (1) Neurotrophic (1) |
1-7mm |
CG PG/LPG DALK PKP |
Graft infection, graft melting, |
1 year |
|
Anitha et al15 |
2022 |
1 |
Fungal keratitis after trauma |
5*5.5mm |
TPG with Gore- tex |
- |
6 months |
The most frequent underlying ocular pathology was infectious keratitis, present in 23 (65.7%) eyes, followed by sterile melts, present in 11 (31.4%) eyes and only one out of the 35 patients, had a 3 mm perforation caused by trauma with a sharp metal ring. 70% of the patients had some form of systemic comorbidity, with diabetes mellitus being the most common in 10 (28.5%) patients. Rheumatoid arthritis was observed in four patients, and three patients had Hepatitis-C association. At the time of presentation, only 6 out of 35 (17.1%) eyes had a clear crystalline lens. 12 out of 35 (34.3%) eyes had lenticular opacities of different grades, while the remaining 17 out of 35 (48.5%) eyes were pseudo-phakic. In all cases, surgeries were uneventful. On day one post op, AC was maintained in all 35 eyes with normal IOP. In one/35 patients, graft dehiscence developed within a week post TPG, who had paracentral corneal perforation of 5.5 mm in size. Despite additional multi-layered amniotic membrane transplantation (AMT) performed as an adjuvant to TPG within a week post-op, the patient developed endophthalmitis, and eventually required evisceration.
In rest 34/35 cases, epithelialization took place within a period of one month and by the end of three months graft was well incorporated within the corneal layers (Figure 3).
During the first three months of follow-up after TPG, two more eyes with central perforation each of 4mm in size developed dehiscence and failed to maintain AC, while one eye with paracentral perforation developed graft ectasia. Managed both cases of graft dehiscence with keratoplasty procedures and addressed graft ectasia by regrafting during this period. Three more patients developed graft ectasia between 3months to 12 months and managed with regrafting in one and keratoplasty procedures in rest two. So, seven out of 35 showed signs of anatomical failure by the end of 12 months follow up. Four more patients required elective keratoplasty procedures for visual rehabilitation between 3 months to 12 months period. So, excluding these 11 patients, at 12-month follow up, 24/35(69%) patients, maintained globe integrity with a well-incorporated scar within the corneal layers (Figure 4) and preoperative vascularization decreased over time after TPG (Figure 8).
During the follow-up period of 3-12 months, seven patients underwent uneventful cataract surgeries. The minimum time interval between TPG and cataract surgeries was six months in all cases. Figure 5 shows the various postoperative complications and Figure 6, Figure 7 depicts different types of additional surgical interventions performed during the entire postoperative period. Additionally, 7 out of 35 patients (20%) developed secondary glaucoma during the follow-up period. All of these cases were of corneal perforation with underlying infective pathology with hypopyon. All these patients were successfully treated with oral and topical AGM. Although the visual recovery was not the primary outcome of our study, but we were able to salvage, improve, and maintain it significantly.
Figure 7
Slit lamp pics of patients undergoing different types of additional surgical intervention during post op period after TPG for managing complications and visual rehabilitation
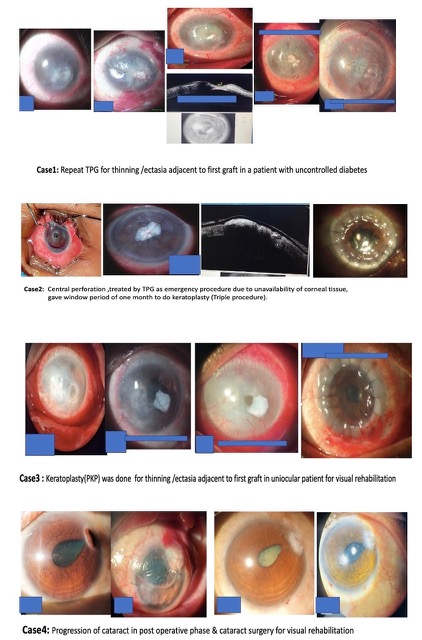
Excluding eight keratoplasty procedures, seven cataract surgeries, and one evisceration, at the 12-month follow-up, out of 35 patients, 19 were evaluated for visual recovery. Of these 19 patients, 13 (68%) showed improvement, as shown in Table 3. Figure 8 shows preoperative, day 1 post-op, and 12-month post-op follow-up photos of patients.
Table 3
Comparative table showing best-corrected visual acuity (BCVA) before and 12 months after TPG for 19/35 patients (16/35 cases of keratoplasty procedures, cataract surgeries, and one evisceration during entire follow up were excluded)
|
Best Corrected Visual Acuity (BCVA) |
Pre op n=19 |
12 months Postop n=19 |
|
H.M.-FC2F |
08 |
|
|
1/60- 2/60 |
06 |
06 |
|
3/60-5/60 |
04 |
09 |
|
6/60-6/24 |
01 |
02 |
|
6/18-6/12 |
02 |
Descriptive analysis was performed using means, medians, ranges, and standard deviations. A bivariate regression was used to identify the preoperative factors associated with the successful outcome of TPG. Rate difference and Odds ratio (OR) with 90% confidence interval (CI) was computed. The SPSS statistical software program (version 13.0, SPSS, Chicago, IL) was used for all analyses. P value < 0.10 was considered as significant.
Discussion
In our study, we observed that the majority of patients fell within the age group of 50-60 years. The reason behind this could be the natural decline of immunity due to ageing and other associated systemic disorders. Similar age groups have also been reported in other studies.16, 22 We found that the most common underlying ocular pathology was infectious keratitis in 23 (65.7%) eyes. This finding is consistent with a study conducted at RPC AIIMS, N. Delhi by Shankar S et al.23 The diabetes mellitus which was the most common 10/35(28.5%) systemic comorbidity in our study group highlighted the fact that diabetic corneal neuropathy could be a cause of non-healing corneal ulcers and complications like perforation.24, 20 Among sterile perforations and descemetoceles, autoimmune association was found in our study and supported by many studies too.18 Three out of 35 of our patients with peripheral pathology (two with perforation and one with descemetocele) had a hepatitis C association. It is well-documented that hepatitis C can lead to corneal perforation.25, 26 In our study group, TPG was commonly done for paracentral pathology such as paracentral perforations in 13 out of 25 (52%) eyes, and paracentral descemetoceles in 8 out of 10 (80%) eyes. However, in a study conducted by Kusumesh et al,22 central perforation was found to be the most common location. This difference may be due to the fact that at our centre, we prefer to perform TPK for central perforation unless a cornea is not available. For instance, in two patients with central perforation of 4 mm in size we performed TPG to buy time until a good quality cornea was obtained. Subsequently, these two patients underwent corneal transplantation. In our study group, in one eye with a 5.5 mm paracentral perforation, the anterior chamber was found to be flat on 7th post-op day, and additional multilayered amniotic membrane transplantation along with cyanoacrylate glue on the edges was done to supplement TPG to rescue the case. In a study by Korah et al., cyanoacrylate glue was used as an adjuvant at the edges for large perforations, resulting in good anatomical success in 74% of cases.17 Unfortunately, in our case, despite this, the patient developed endophthalmitis, and evisceration had to be performed. After experiencing this failure, we decided to avoid performing TPG in cases of paracentral perforation larger than 5 mm. Two eyes with central perforation, each of 4mm size, were by timely done TPG as emergency procedure in the absence of corneal tissue. TPG has been shown to be a very successful modality in managing corneal perforations/descemetoceles in various studies with different follow up time period summarised in Table 2.15, 18, 22, 23, 24, 20, 25, 26, 17
Our study also had similar and comparable success rate. In our study, AS-OCT was used to monitor the characteristic changes in the graft over time. An invitro study conducted by Morris DA et al. has shown that tenon fibroblasts play an important role in scar formation. This property of tenon’s capsule is used to restore the desired thickness in the area of corneal thinning or perforation. A stable ocular surface with a corneal scar, with or without vascularization, can be achieved by the third postoperative month.10, 22 Our study also found that the postoperative course of diabetic patients was unpredictable. We noticed that all four cases of graft ectasia that occurred within six months of follow-up were in diabetic patients. This result may be due to delayed wound healing in diabetic patients.24, 20 Though TPG is generally an emergency procedure in the unavailability of corneal tissue and requires cornea transplant as a definite surgery at a later date but in our study, only 8/35(23%) patients required keratoplasty procedures during the entire 12-month period. This is because we mainly performed TPG for paracentral and peripheral perforation/descemetocele, (92% and 90% respectively) where the final scar did not affect the vision of the patient directly. Secondly, in our study, we took into account the patient's systemic comorbidity, such as diabetes or autoimmune diseases. If the comorbidity was not well controlled and the patient had ambulatory vision in the eye, then we preferred to do a repeat TPG instead of a high-risk keratoplasty, even if ectasia developed. Our findings were different from Kusumesh et al group, who had to register more than 70% of patients for keratoplasty procedures, probably because they did TPG in the central perforation more.22
The limitation of our study was short follow up period of one year only.
Conclusion
Based on our experience, we have found that TPG is an effective, simple, and inexpensive technique for managing corneal perforations and descemetoceles ranging in size from 2-5mm. Eyes becomes stabilized within one month post TPG. In paracentral and peripheral perforation/descemetocele, the need for keratoplasty can be omitted with good tensile strength over graft area. In central perforation where corneal tissue was not readily available, TPG preserved the eyes and allowed for a few weeks' time to perform keratoplasty with good quality donor cornea.