- Visibility 276 Views
- Downloads 72 Downloads
- Permissions
- DOI 10.18231/j.ijceo.2024.008
-
CrossMark
- Citation
Does ranibizumab biosimilars fare as well in macular neovascularisation?
- Author Details:
-
Manoj Soman
-
Sameer Iqbal *
-
Indu J Nair
-
Ravi R V
-
Unnikrishnan Nair
Abstract
Purpose: To evaluate the effectiveness and safety profile of Ranibizumab Biosimilar in Macular Neovascularisation and compare outcomes with patent Ranibizumab.
Materials and Methods: A retrospective analysis during the period 2017-2020 was conducted at a tertiary eye care centre in South Kerala on patients with wet AMD who had received Razumab, a biosimilar of Ranibizumab as a loading dose followed by PRN schedule. This was compared to a similar data obtained from patented Ranibizumab (Lucentis) during the same period. Endpoints analyzed included improvement in BCVA, proportion of patients with persistent IRF/SRF and complications at 1 year.
Results: Of 164 eyes analyzed 76 eyes received Razumab and 88 eyes received Lucentis with 32% and 50% males in each group. After the loading dose both drugs showed similar efficacy and the final vision (median logmar; 0.24 vs 0.17; p 0.189), presence of residual CME (31.6% vs 18.2%; p 0.469) and SRF (57.9% vs 61.9%; p 0.796) were similar in both groups at 12 months. The mean number of injections was 8 in the Razumab group comparable to Lucentis (6.4). Acceptability and less drop outs were seen in Razumab patients compared to Lucentis. Though some eyes developed mild uveitis (4.3%) with one of the initial batches of Razumab, it was not evidenced later. No other safety concerns or side effects were reported with the biosimilar.
Conclusions: The Biosimilar Razumab is as effective as the patent molecule in reducing macular fluid and improving visual acuity in patients with macular neovascularization over 1 year on a PRN schedule. Being cheaper it is a safe alternative for patients who often need long term management.
Introduction
Age-related macular degeneration is a common cause of visual impairment in older population.[1] Exudative AMD is characterized by the formation of CNV membranes that spreads to the sub-RPE or sub-retinal space resulting in intra-retinal fluid (IRF) or sub-retinal fluid (SRF) accumulation leading to blurring of vision.[2]
Vascular endothelial growth factor (VEGF)-A is believed to play a major role in the pathogenesis of wet age-related macular degeneration and macular edema.[3], [4] Hence Anti-VEGF agents including ranibizumab have been found to be effective in management of exudative AMD. Ranibizumab is a humanized monoclonal antibody that binds to the VEGF-A isoforms and prevents it from interacting with the receptor on the endothelial cells.[5] Razumab is the world’s first biosimilar to ranibizumab developed by Intas Pharmaceuticals Limited, India. It was approved by the Drug Controller General of India for management of wet AMD, diabetic macular edema and macular edema secondary to retinal vein occlusions. In this study we tried to evaluate the effectiveness and safety profile of Razumab in Macular Neo-vascularization and compared its treatment outcomes with that of patent Ranibizumab.
Materials and Methods
A retrospective analysis of data of patients with wet AMD who had received Razumab, a biosimilar of Ranibizumab as a loading dose followed by PRN schedule during a period from March 2017 to April 2020 was done. Study was conducted at a tertiary eye care centre in South Kerala after protocol being approved by institutional ethical committee. This was then compared to a similar data obtained from AMD patients who have been treated with patented Ranibizumab (Lucentis) during the same period. Endpoints analyzed included improvement in BCVA, proportion of patients with persistent IRF/SRF and complications at the end of 1 year.
Both treatment naive and previously treated patients with anti VEGF were included in the study. Those with a minimum follow up of one year were only included in this study. All patients underwent a detailed eye examination that included slit-lamp biomicroscopy, tonometry, fundus examination with IDO, fluorescein angiography, indocyanine green angiography and OCT whenever indicated. Patients were excluded if they had coexisting significant media opacities which limited the ability to take good images or if they had other suspected causes for CNVM. Razumab PRN treatment regimen was guided by disease activity criteria as evident with increase in IRF/SRF in OCT or active leakage from the CNVM as compared to previous visit. Treatment was discontinued if no disease activity was noted. Patients diagnosed to have active CNVM were treated with loading doses (injection monthly for first 3 month) of intravitreal anti-VEGF injection (Razumab or Ranibizumab 0.5mg in 0.05 ml) followed by PRN basis. After loading dose, patients were reassessed for evidence of activity with SD-OCT primarily and ICGA was done whenever indicated. At each monthly visit, OCT was repeated and presence of fluid (IRF/SRF) and modification of PED on OCT were recorded. Retreatment was based on standard guidelines using clinical and OCT criteria. The safety endpoints were occurrence of any adverse events including any hypersensitivity reactions or significant intraocular inflammation post injection.
Results
164 patients with neovascular AMD were included in our study. Of 164 eyes analyzed 76 eyes received Razumab ([Figure 1]) and 88 eyes received Lucentis (ranibizumab) ([Figure 3]) with 32% and 50% males in each group. Mean age of study group was 67.27 ± 8.14 in ranibizumab treated group and 69.53 ± 9.12 in Razumab treated group. Common associated systemic risk factors were diabetes mellitus (31.4%), hypertension (43%) and ischemic heart disease (15.7%). Baseline variables and demographic data of both the groups are given in [Table 1]. After the loading dose both drugs showed similar efficacy as shown in [Table 2] and the final vision (median logmar; 0.24 vs 0.17; p 0.189), presence of residual CME (31.6% vs 18.2%; p 0.469) and SRF ([Figure 2]) (57.9% vs 61.9%; p 0.796)was similar in both groups at 12 months ([Table 4]). The mean number of injections was 8 in the Razumab group comparable to Lucentis (6.4). Acceptability and less drop outs were seen in Razumab patients compared to Lucentis. Though some eyes developed mild uveitis (4.3%) with one of the initial batches of Razumab, it was not evidenced later. No additional safety concerns, either ocular or systemic were noted with the biosimilar.
|
Baseline Variables |
Ranibizumab group (N=88) |
Razumab (N=76) |
P value |
||
|
Age |
Mean ± SD |
67.27 ± 8.14 |
69.53 ± 9.12 |
0.413 |
|
|
Gender |
Males |
Percentage Median (IQR) |
50 |
31.6 |
0.233 |
|
Females |
50 |
68.4 |
|||
|
Eye |
Left Eye |
54.5 |
31.6 |
0.209 |
|
|
Right Eye |
45.5 |
68.4 |
|||
|
Previous ANTIVEGF |
0 (0 - 0) |
5 (2 - 8) |
<0.001 |
||
|
BaselineVn LOGMAR |
0.47 (0.27 - 0.5) |
0.48 (0.18 - 0.6) |
0.979 |
||
|
IOP |
Mean ± SD |
14.23 ± 3.66 |
12.53 ± 2.82 |
0.101 |
|
|
Lens Status |
PP |
Percentage |
22.7 |
36.8 |
0.322 |
|
Phakic |
77.3 |
63.2 |
|||
|
OCT SRF |
90.9 |
78.9 |
0.28 |
||
|
OCT CME |
3 (13.6) |
11 (57.9) |
0.007 |
||
|
SRF Height |
Median (IQR) |
173.5 (106 - 252.75) |
133 (32 - 172) |
0.139 |
|
|
Choroidal Thickness |
Mean ± SD |
348.27 ± 42.19 |
270.42 ± 59.71 |
<0.001 |
|
At 4 months |
Ranibizumab group (N=88) |
Razumab (N=76) |
P value |
|
|
LOGMAR |
Median (IQR) |
0.17 (0.17 - 0.17) |
0.18 (0 - 0.34) |
0.418 |
|
IOP |
Mean ± SD |
13.82 ± 3.63 |
12.53 ± 2.35 |
0.189 |
|
OCT SRF |
Percentage |
36.4 |
52.6 |
0.295 |
|
OCT CME |
4.5 |
31.6 |
0.036 |
|
|
SRF Height |
Median (IQR) |
0 (0 - 125.25) |
63.5 (0 - 238) |
0.271 |
|
At 6 months |
Ranibizumab group (N=88) |
Razumab (N=76) |
P value |
|
|
LOGMAR |
Median (IQR) |
0.17 (0.17 - 0.43) |
0.24 (0.13 - 0.34) |
0.364 |
|
IOP |
Mean ± SD |
13.27 ± 2.81 |
12.74 ± 2.53 |
0.525 |
|
OCT SRF |
Percentage |
40.9 |
52.6 |
0.453 |
|
OCT CME |
22.7 |
31.6 |
0.524 |
|
|
SRF Height |
Median (IQR) |
0 (0 - 111.25) |
18 (0 - 103.75) |
0.402 |
|
At 12 months |
Ranibizumab group (N=88) |
Razumab (N=76) |
P value |
|
|
LOGMAR |
Median (IQR) |
0.17 (0.17 - 0.30) |
0.24 (0.13 - 0.34) |
0.189 |
|
IOP |
Mean ± SD |
12.82 ± 2.38 |
13.78 ± 2.46 |
0.222 |
|
OCT SRF |
Percentage |
61.9 |
57.9 |
0.796 |
|
OCT CME |
18.2 |
31.6 |
0.469 |
|
|
SRF Height |
Median (IQR) |
92.5 (0 - 171.25) |
72.5 (0 - 129.25) |
0.713 |
Statistical analysis was carried out with SPSS 23.0 version. The unpaired t-test and Mann Whitney U test were used to analyze the continuous variables with normal distribution and those without normal distribution respectively. Paired variables were analyzed using Paired T test and Wilcoxon signed rank test. Categorical variables were analyzed using Chi square test or Fisher exact test. Paired categorical variables were analyzed utilizing McNemar test. Variables with p value <0.05 was considered as statistically significant.
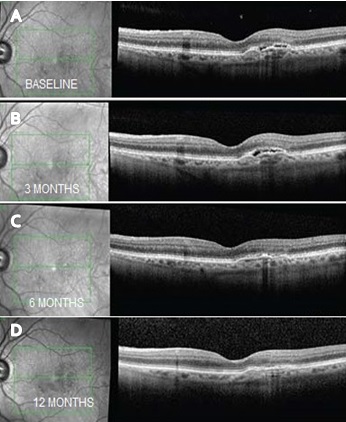
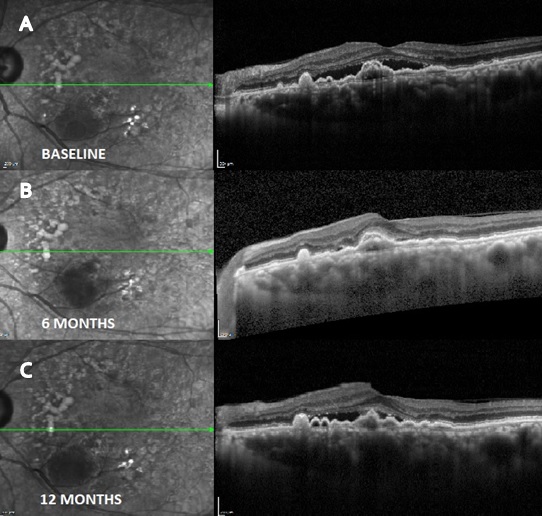
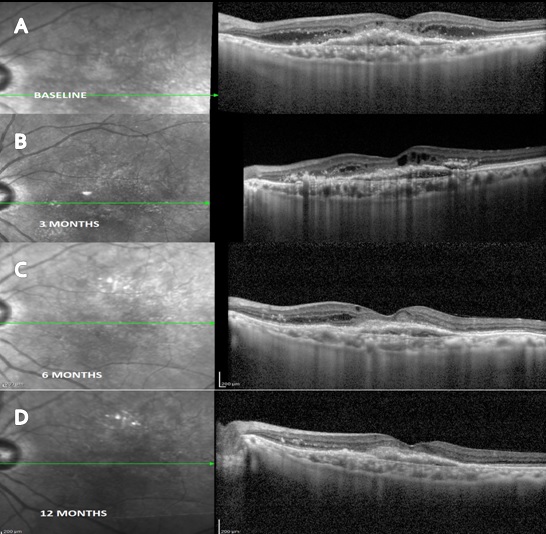
Discussion
Anti-VEGF agents have dramatically changed the management of exudative AMD which results in severe visual loss if untreated. As compared to the previous treatment techniques like Photo Dynamic Therapy and Thermal laser treatment, these agents significantly improve visual prognosis and outcomes nAMD patients.[6]
Main aim of our study was to evaluate the effectiveness and safety profile of Ranibizumab Biosimilar (Razumab) in macular neo-vascularization and to compare its outcomes with patent Ranibizumab (Lucentis). Efficacy of ranibizumab in wet AMD patients has been well established in two previous pivotal phase 3 studies – MARINA[7] and ANCHOR.[8] The FDA approval of Ranibizumab for nAMD was based on these studies which showed visual acuity improvement over 1 year time with monthly dosing. Similarly, the efficacy and tolerability of Razumab, a biosimilar to ranibizumab have been demonstrated recently in a few multi-centric, randomized, prospective studies.[9], [10] Multicentric retrospective RE-ENACT and RE-ENACT 2 studies demonstrated the significant improvement in various parameters of wet AMD patients including BCVA, CFT, IRF and SRF with monthly injections of razumab.[10], [11]
Our study showed that the Razumab biosimilar is just as efficient as the patent Ranibizumab when it is used to treat nAMD at any four-week interval. Both the adjusted treatment difference between the treatment groups for change from baseline in BCVA (median logmar;0.24 vs 0.17; p 0.189) and presence of residual CME (31.6% vs 18.2%; p 0.469) and SRF (57.9% vs 61.9%; p 0.796)was similar at 12 months. This was consistent with the study conducted by SJ Woo et al. even though their evaluation of BCVA from baseline was at 8 weeks and CST from baseline was at 4 weeks duration.[12]
In our study, patients were dosed on PRN basis after loading phase and the safety and efficacy of Razumab were assessed after 1 year duration. Visual acuity improvement of nAMD patients has been reported in ANCHOR and MARINA studies after monthly injections of anti VEGF agents (ranibizumab).[7], [8] These studies found significant improvement in BCVA by 3 months and patients could sustain this improvement till the end of treatment period of 24 months. The efficacy of ranibizumab biosimilar was assessed using sensitive endpoints such as change in visual acuity and presence of residual CME and SRF revealed by SD-OCT. Both the groups showed similar efficacy in improving the BCVA and also in reducing SRF/IRF at the end of 4, 6 and 12 months follow up evaluations.
The mean number of injections was 8 in the Razumab group when compared to Lucentis (6.4) at the end of 12 months. Acceptability and less drop-outs were seen in Razumab patients. Increase in intraocular pressure is considered to be among the common adverse effects. But the mean changes in IOP during course of our study period were minimal and was insignificant (p value >0.05), similar to previous reports in literature.[13] Other common adverse effects include subconjuctival haemorrhage, floaters, visual disturbances, intraocular inflammation, ocular pain, and ocular hyperemia. Though some eyes developed mild uveitis (4.3%) with one of the initial batches of Razumab, it was not evidenced later during our study period. Non-ocular side effects such as hypertension, nasopharyngitis, headache and thromboembolic events have also been reported.[14], [15], [16] No additional safety concerns, either ocular or systemic, were noted with the Razumab treated patients.
The generalizability of the results from our study is upheld by its consistency with previous research on ranibizumab. In particular, mean changes from baseline in BCVA at 6 months were 7.4 letters compared with 6.5 letters in the MARINA study, 10.6 letters in ANCHOR study and 6.6 letters in the CATT study trials. In real world scenario, it is possible that nAMD patients are undertreated and do not receive as many anti-VEGF injections as recommended, which could result in lower efficiency than what is observed in clinical trial settings.[17] The use of biosimilar can add to the cost savings, making it more affordable for general population. Thus, a biosimilar version of Ranibizumab like Razumab may reduce few limitations currently imposed by health care providers or payors, which would allow patients to have a better opportunity for receiving an effective treatment regimen.
Our study limitations included the unavailability of details regarding the pattern, size and type of CNVMs. Additionally, the data on patients who had obtained therapies earlier were not captured, meaning that information on "treatment naïve" versus "previously treated patients" could not be provided.
Conclusions
Razumab, a ranibizumab biosimilar showed equivalent efficacy compared with ranibizumab in participants with nAMD in improving visual acuity and in decreasing CME and SRF over 1 year on a PRN schedule. Besides, being cheaper and with similar safety profile of ranibizumab, it forms an effective alternative for patients who often need long term management. Therefore, a safer, cheaper and effective biosimilar like razumab may decrease several cost related restrictions of patent ranibizumab. This will allow patients suffering from nAMD to have a better opportunity of getting an effective treatment regimen.
Source of Funding
None.
Conflict of Interest
None.
References
- Lim L, Mitchell P, Seddon J, Holz F, Wong T. Age-related macular degeneration. Lancet. 2012;379(9827):1728-38. [Google Scholar]
- Jaffe G, Martin D, Toth C, Daniel E, Maguire M, Ying G. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120(9):1860-70. [Google Scholar]
- Grisanti S, Zhu Q, Tatar O, Lueke J, Lueke M, Tura A. Differential expression of vascular endothelial growth factor-a isoforms in neovascular age-related macular degeneration. Retina. 2015;35(4):764-72. [Google Scholar]
- Gupta S, Yadav I, Deshmukh S, Maurya R, Singh V. Predictors of visual response to Intravitreal Bevacizumab for treatment of Diabetic Macular Edema. Indian J Clin Exp Ophthalmol. 2015;1(1):35-40. [Google Scholar]
- Hernandez-Pastor L, Ortega A, Garcia-Layana A, Giraldez J. Ranibizumab for neovascular age-related macular degeneration. Am J Health Syst Pharm. 2008;65(19):1805-14. [Google Scholar]
- Deonandan R, Jones S. Anti-Vascular Endothelial Growth Factor Drugs for the Treatment of Retinal Conditions: A Review of the Safety. . 2017. [Google Scholar]
- Rosenfeld P, Brown D, Heier J. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;14(14):1419-31. [Google Scholar]
- Brown D, Kaiser P, Michels M. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432-44. [Google Scholar]
- Sameera V, Ayachit A, Joshi S, Guruprasad A. Safety and efficacy of Razumab-the new biosimilar in India: our experience. Kerala J Ophthalmol. 2016;28(3):180-5. [Google Scholar]
- Sharma S, Khan M, Chaturvedi A. RE-ENACT Study Investigators Group. Real-life clinical effectiveness of Razumab_ (the world’s first biosimilar of ranibizumab) in retinal vein occlusion: a subgroup analysis of the pooled retrospective RE-ENACT study. Ophthalmologica. 2019;241(1):24-31. [Google Scholar]
- Sharma A, Reddy P, Kuppermann B, Bandellof F, Lowenstein A. Biosimilars in ophthalmology: “is there a big change on the horizon?. Clin Ophthalmol. 2018;12:2137-43. [Google Scholar]
- Woo SJ, Veith M, Hamouz J, Ernest J, Zalewski D, Studnicka J. Efficacy and Safety of a Proposed Ranibizumab Biosimilar Product vs a Reference Ranibizumab Product for Patients With Neovascular Age-Related Macular Degeneration: A Randomized Clinical Trial. JAMA Ophthalmol. 2021;139(1):68-76. [Google Scholar]
- Sharma S, Khan M, Chaturvedi A. A Multicenter, Retrospective Study (RE-ENACT 2) on the Use of Razumab™ (World's First Biosimilar Ranibizumab) in Wet Age-Related Macular Degeneration. Ophthalmol Ther. 2020;9(1):103-14. [Google Scholar]
- Rosenfeld P, Brown D, Heier J, Boyer D, Kaiser P, Chung C. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-31. [Google Scholar]
- Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingemann RO, Axer-Siegel R. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011;118(5):831-840. [Google Scholar]
- Silva R, Axer-Siegel R, Eldem B, Guymer R, Kirchhof B, Papp A. The SECURE study: long-term safety of ranibizumab 0.5 mg in neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):130-139. [Google Scholar]
- Holz F, Bandello F, Gillies M. Safety of ranibizumab in routine clinical practice: 1-year retrospective pooled analysis of four European neovascular AMD registries within the LUMINOUS programme. Br J Ophthalmol. 2013;97(9):1161-7. [Google Scholar]
How to Cite This Article
Vancouver
Soman M, Iqbal S, Nair IJ, V RR, Nair U. Does ranibizumab biosimilars fare as well in macular neovascularisation? [Internet]. Indian J Clin Exp Ophthalmol. 2024 [cited 2025 Oct 28];10(1):50-54. Available from: https://doi.org/10.18231/j.ijceo.2024.008
APA
Soman, M., Iqbal, S., Nair, I. J., V, R. R., Nair, U. (2024). Does ranibizumab biosimilars fare as well in macular neovascularisation?. Indian J Clin Exp Ophthalmol, 10(1), 50-54. https://doi.org/10.18231/j.ijceo.2024.008
MLA
Soman, Manoj, Iqbal, Sameer, Nair, Indu J, V, Ravi R, Nair, Unnikrishnan. "Does ranibizumab biosimilars fare as well in macular neovascularisation?." Indian J Clin Exp Ophthalmol, vol. 10, no. 1, 2024, pp. 50-54. https://doi.org/10.18231/j.ijceo.2024.008
Chicago
Soman, M., Iqbal, S., Nair, I. J., V, R. R., Nair, U.. "Does ranibizumab biosimilars fare as well in macular neovascularisation?." Indian J Clin Exp Ophthalmol 10, no. 1 (2024): 50-54. https://doi.org/10.18231/j.ijceo.2024.008
