- Visibility 126 Views
- Downloads 24 Downloads
- DOI 10.18231/j.ijceo.2023.013
-
CrossMark
- Citation
How do polypoidal choroidal vasculopathy patients fare long term - A real world data
- Author Details:
-
Manoj S
-
Asmita Indurkar
-
Sameer Iqbal *
-
Ravi R V
-
Mancy Mathew
-
Unnikrishnan Nair
Introduction
Polypoidal choroidal vasculopathy (PCV) was first described by Yanuzzi in 1982.[1] The current gold standard for diagnosing PCV is by indocyanine green angiography (ICGA). PCV has a various range of phenotypic features with eyes in this group demonstrating increased choroidal thickness and/or the presence of pachyvessels.[2]
Major trails like EVEREST study[3] compared combination therapy versus monotherapy with ranibizumab while PLANET study[4] compared results of aflibercept with and without rescue therapy. Long-term outcomes of various management options in real world have been reported.[5] Our present study aims to know the biomarkers predicting the long-term outcomes of PCV in Indian population and evaluate various factors that correlate with better visual outcomes in PCV patients.
Materials and Methods
This is a long term retrospective study of 51 patients diagnosed with polypoidal choroidal vasculopathy (PCV) with a minimum follow up of five years. The InstitutionalEthical Committee approved the research project. PCV was diagnosed based on the EVEREST study criteria ie. the presence of subretinal focal ICGA hyper-fluorescence and at least one of the following angiographic or clinical criteria: Branching Vascular Network (BVN), pulsatile polyp, nodular appearance when viewed stereoscopically, and presence of hypofluorescent halo.[3]
The inclusion criteria were patients diagnosed with PCV with a minimum of five years follow up. The exclusion criteria were patients previously treated with Photodynamic therapy (PDT) or intravitreal injections of anti-VEGF or patients with any other ocular pathology.
Systemic co-morbidities and associated ocular pathologies were recorded. Patients involved in the study underwent comprehensive ophthalmic examinations, including best-corrected visual acuity (BCVA), fundus photography, OCT (Spectralis, Heidelberg Engineering, Germany), FA and ICGA (Spectralis, Heidelberg Engineering), and OCT Angiography (Spectralis). Clinical diagnoses were made by fundus photography, OCT, and FA/ICGA findings by retina specialists.
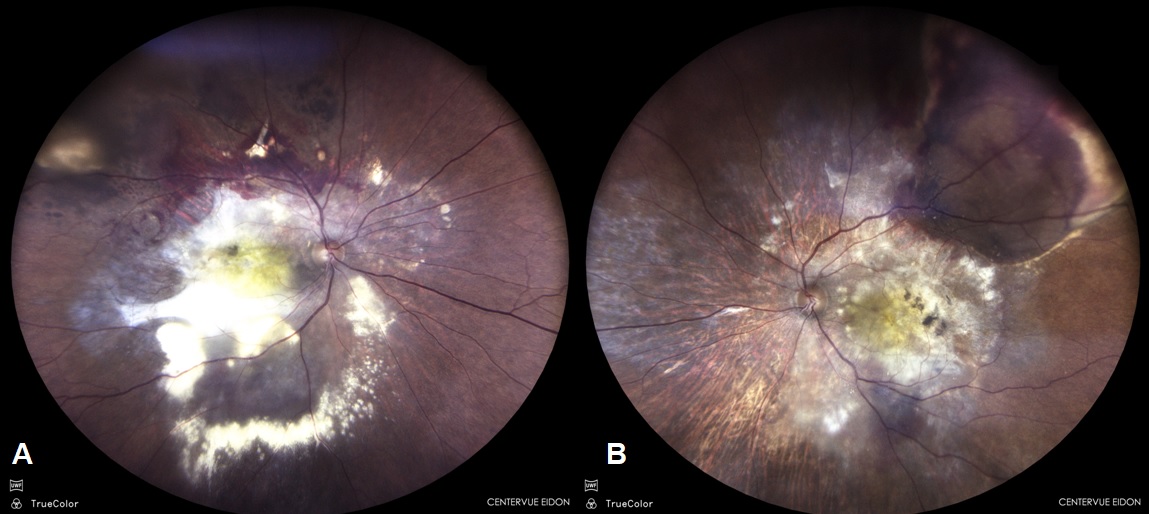
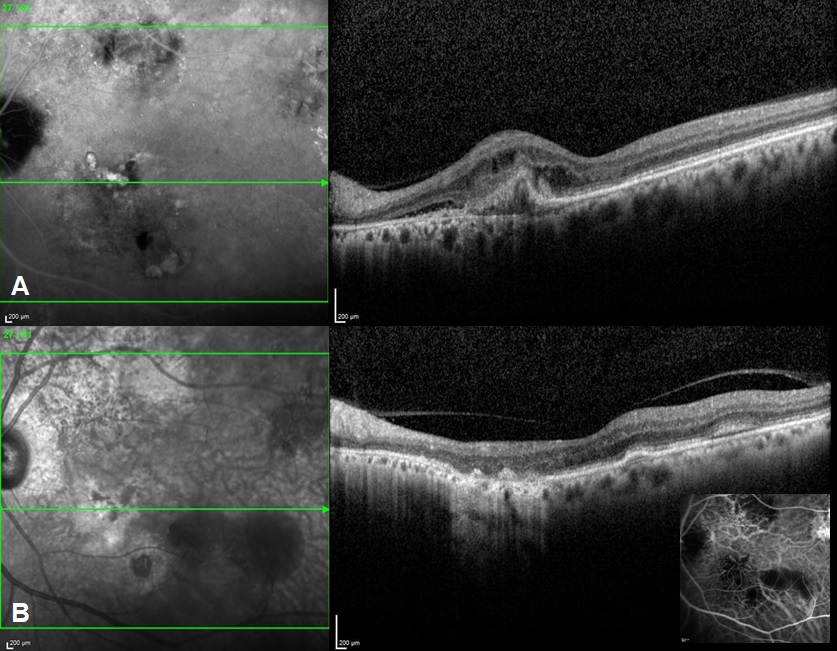
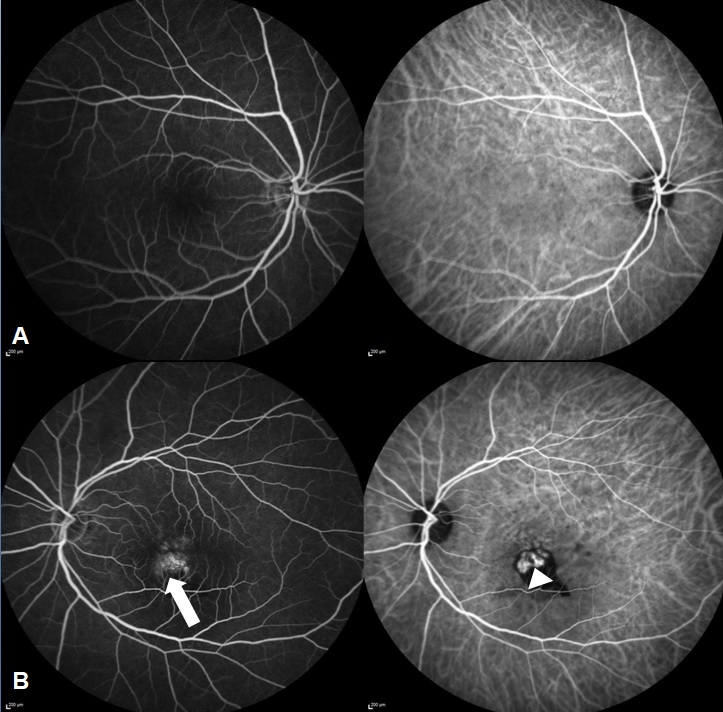
Fundus evaluation was done and specific features like orange nodules, haemorrhage, exudates were noted ([Figure 1]). Various OCT features were recorded like the type of Pigment epithelial detachment, sub RPE ring like lesion and double layered sign (DLS). Maximum pigment epithelial detachment (PED) height, width and area and maximum sub retinal fluid (SRF) height was compared at the baseline and at five years. Subfoveal Choroidal thickness, presence of pachyvessels and choroidal thickness at the level of pachyvessels were recorded. FFA-ICGA was done at baseline and at follow-ups ([Figure 2]). Number of polyps, pulsating polyps, type of network and type of PCV were noted. This was compared with ICGA at 5 years.
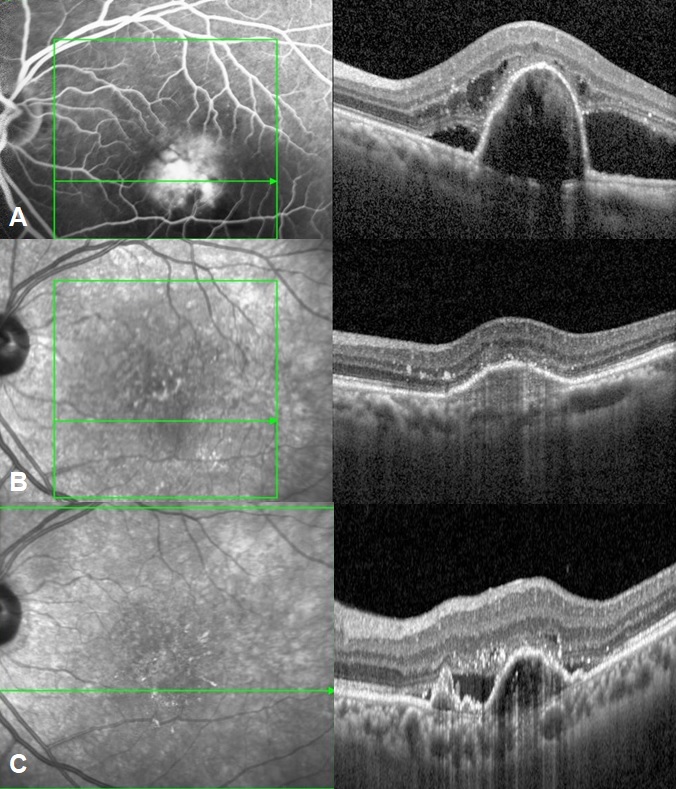
OCTA when done was evaluated for network type and polyps seen. Treatment in the form of anti-VEGF and Photodynamic therapy, rescue and primary was noted. Treatment regimen varied depending on the preference of treating physician with either the “as needed” or pro re nata (PRN) or treat-and-extend (TAE) regimens adopted. Treatment regimen, and intra-vitreal anti-VEGF agent was decided by the treating physician. The anti VEGF switch and the number of injections before switching were noted. Visual acuity and number of injections required at baseline and at each year were recorded. Post treatment complications were noted.
The primary outcomes were the mean visual acuity (VA) changes from baseline. Significant vision loss was defined as losing 10 letters from baseline at the point of the follow-up. Secondary outcomes included the change in the maximum height, area and width of the PED, change in the size of network and polyps, mean number of injections and the number of eyes where the treatment was switched, response of PCV based on its features.
Compliance of the patient each year was noted. Compliance was defined as completing the follow-up until the end of the observation period, regardless of the yearly injection count or treatment switching. Reasons for compliance were enumerated.
Result
Baseline demographic features of our study population are explained in [Table 1]. Total of 128 eyes of PCV were analyzed from our patient records, out of which 51 eyes had a follow up of five years and were selected retrospectively for the study. The mean age of the 51 patients was 66 years, with 32 males (63%) and 19 females (37%). Of the 45 PCV eyes, 76.4% lesions occurred predominantly at the macula, while 23.5% were peripapillary lesions. The clinical presentations were variable with exudates seen in 23 eyes (45%) though visible polyps 8 eyes (16.6%) and massive sub retinal hemorrhage 9 eyes (17.7%) were less frequent.
Baseline OCT features of study population are demonstrated in . Double layer sign was seen in 45 eyes (88.8%) with thumb shaped PED seen in 30 eyes (58.8%). Sub retinal fluid was the presenting sign in 42 eyes (82.3%) while 17 eyes (33.3%) also had intraretinal fluid. Diffuse PED were noted in 26 eyes (50.9%), hemorrhagic PEDs in 14 eyes (27.4%) and intra retinal exudates were noted in 28 eyes (54.0%). DLS with diffuse PEDs or thumb shaped PEDs were the most common finding while Sub RPE ring like lesion (17.7%) was less frequent on SD-OCT. Mean choroidal thickness of 51 eyes was 290.4 microns. 23 eyes showed presence of a pachyvessel associated with the lesion and mean choroidal thickness at the level of pachyvessel was 333.2 microns. Mean choroidal thickness at the end of five years was 288 microns while the mean choroidal thickness at the level of pachyvessels was 301.1 microns.
[Table 2], [Table 3] compare imaging characteristics of PCV patients at baseline and after a 5-year follow-up. At the baseline, mean maximum SRF height was 163 microns while the mean maximum PED height was 420.7 microns. Mean maximum basal diameter of the largest PED was 2327.5 microns. Mean maximum planar area of the largest PED was 0.62 microns sq while, at the end of 5 years, mean maximum SRF height was 94.47 microns, mean maximum PED height was 300.9 microns, mean maximum basal diameter of the largest PED was 2486.1 microns and mean maximum planar area of the largest PED was 0.43 microns sq.
At baseline, 45 eyes showed polyps on indocyanine green angiography ([Figure 3]) amongst which 19 polyps showed pulsations in video angiography. In 8 eyes presence of polyp could not be estimated due to blocked fluorescence in ICGA. Majority of our eyes 37 eyes (72.5%) were type 1 PCV ie. PCV with apparent branching vascular network while type 2 PCV (PCV without an apparent BVN) was seen in 14 eyes (27.4%). OCTA was done in 12 eyes. 8 eyes showed polyps and 21 eyes showed a well-defined network in the avascular slab which was larger in maximum dimensions than the network detected in ICGA. SD OCTA was sensitive in identifying BVNs mostly detecting larger networks compared to ICGA but was poor in identifying polyps and demonstrated segmentation artifacts.
Treatment details of study population are described in . 34 eyes received monotherapy of anti-VEGF injections, and only 17 eyes received the combination of anti-VEGF injection and PDT at the time of the first treatment ([Figure 4]). 4 eyes received repeat PDT in a mean of two years. The mean number of injections was 18.7 (21.5%). 11 eyes received primary PDT while (11.7%) 6 eyes received rescue PDT. Of the patients who received PDT, low fluence PDT was as effective as standard fluence. Though not significant, PDT eyes required lesser injections than monotherapy (6.1 injections vs 8.3 injections), though compliance in both the groups was not the same. Anti VEGF switch was done in 28 eyes after a mean of 3.9 injections. The mean follow-up period prior to switching was 1.8 years. 11 eyes were switched to ranibizumab out of which 7 were later switched to aflibercept, while 17 eyes were directly switched to aflibercept injection. Mean number of injections in the first year were 2.6 and in the second year were 1.7. Mean number of injections in the year 3 to 5 was 4.4. As an agent aflibercept seemed to be better than ranibizumab and was the most preferred switching agent (55% needed switch). Switching from ranibizumab to aflibercept was significantly more frequent. Loading dose followed by PRN was the only feasible regimen with relatively reasonable compliance. The mean VA when the treatment was switched was 0.50 log MAR and mean VA at 1 year after switching was 0.32 log MAR, and did not differ significantly(p = 0.3).
Recurrence was defined as reactivation if monthly injections were discontinued after an anatomical response to anti-VEGF injections. Resistance/recalcitrance was defined as persistence or increase in the intra/subretinal fluid, pigment epithelial detachment (PED) or hemorrhage after minimum three injections on a monthly basis. Retreatment was applied when new retinal hemorrhage, visual acuity loss with intraretinal fluid, or subretinal fluid were observed without treatment. Recurrence was noted in 29 eyes (56.8%) and most commonly noted at the end of 14 months, no significant difference was found between various treatment regimens or type of PCV. The mean BCVA improved from 0.42 to 0.6 log mar. Improvement in vision was achieved in 42% eyes, while visual loss occurred in 26.6% eyes, the deterioration more in type 1 PCV compared to type 2 ([Table 5]). Mean VA in type 1 and type 2 at baseline was log mar 0.54 and 0.52 respectively whereas at 5 year follow-up was 0.48 and 0.40. Baseline good vision, thicker CCT, smaller lesions and minimum recurrences at year 1 were the factors associated with good outcome. The most common reasons for poor compliance included no insurance (16 patients) or family support (4patients). Although the completion rate of 1 year was as high as 86.3% the final 5-year follow-up rates were 40.1%. Most common complications noted in our study group was RPE rip seen in 6 eyes (11.7%) whereas 2 eyes (3.9%) required surgical intervention for breakthrough vitreous hemorrhage.
|
At baseline |
Number of patients/eyes (%) |
|
Demography |
|
|
Mean Age |
66 years |
|
Males |
32(63%) |
|
Females |
19(37%) |
|
Right eye |
34 |
|
Left eye |
17 |
|
Location of lesion |
|
|
Macular |
39(76.4%) |
|
Peripapillary |
12(23.5%) |
|
Clinical features |
|
|
Sub retinal haemorrhage |
9(17.7%) |
|
Orange red nodules |
8(16.6%) |
|
Exudates |
23(45%) |
|
Type of PCV |
|
|
Type 1 |
37 (72.5%) |
|
Type 2 |
14 (27.4%) |
|
OCT features |
Baseline |
At 5 years |
|
|
Number of eyes/% |
|
|
DLS |
45(88.8%) |
45(88.8%) |
|
Thumb shaped PED |
30(58.8%) |
9(4.5%) |
|
Subretinal Fluid |
42(82.3%) |
28(54.9%) |
|
Intraretinal Fluid |
17(33.3%) |
7(13.7%) |
|
Diffuse PED |
26(50.9%) |
28(54.9%) |
|
Haemorrhagic PED |
14(27.4%) |
0 |
|
Intraretinal exudates |
28(54%) |
17(33.3%) |
|
Sub RPE ring like lesion |
9(17.7%) |
3(5.8%) |
|
Peaked PED |
17(33.3%) |
5(9.8%) |
|
Multilobular |
20(39.2%) |
25(49.0%) |
|
Scar/RPE atrophy |
- |
8(15.6%) |
|
ORT |
- |
2(3.9%) |
|
|
At Baseline |
At 5 years |
|
Mean maximum SRF height |
163 microns |
94.47 microns |
|
Mean maximum PED height |
420.7 microns |
300.9 microns |
|
Mean maximum PED base diameter |
2327.5 microns |
2486.1 microns |
|
Mean maximum PED planar area |
0.62 microns sq |
0.43 microns sq |
|
Mean central choroidal thickness |
290.4 microns |
288 microns |
|
Mean choroidal thickness at level of pachyvessel |
333.2 microns |
301.1 microns |
|
Number of polyps on ICGA |
45 eyes (88.2%) |
9 eyes (17.6%) |
|
Number of pulsating polyps |
19 eyes (37.2%) |
4 eyes (7.8%) |
|
Mean maximum dimension of the network on ICGA |
2445 microns |
1973 microns |
|
Mean maximum dimension of the network on OCTA |
2621 microns |
2189 microns |
|
Treatment |
Number of eyes/% |
|
Anti VEGF monotherapy |
34 eyes (66.6%) |
|
Combination therapy |
17 eyes (33.3%) |
|
Primary PDT |
11 eyes (21.5%) |
|
Rescue PDT |
6 eyes (11.7%) |
|
Repeat PDT |
4 eyes (7.8%) |
|
Anti VEGF switch |
28 eyes (54.9%) |
|
Low fluence PDT |
6 eyes (11.7%) |
|
Standard fluence PDT |
11 eyes (21.5%) |
|
|
Type 1 |
Type 2 |
|
Mean Baseline BCVA |
0.54 |
0.52 |
|
Mean BCVA at 5 years |
0.48 |
0.40 |
|
Number of injections |
7.2 |
6.8 |
|
Combination therapy |
6 eyes |
11 eyes |
Discussion
The present study was conducted to analyze various factors affecting the treatment outcome of Polypoidal choroidal vasculopathy and its long term clinical course. Though factors like baseline visual acuity, presence of sub-retinal fluid and the maximum lesión dimension have been the predictors of visual acuity in PCV, there are very few studies available in literature that study impact of these factors in predicting long term treatment outcomes specifically in Indian population.
Recently, OCT has become an important modality to diagnose PCV. Sub RPE ring like lesions are one of the important criteria in OCT diagnosis of PCV as evident in many studies.[2] However, the present study did not find it to be a common finding on OCT. Instead, most common finding on OCT in our study was DLS and thumb shaped PED. Both of these OCT findings are known to have high sensitivity in the diagnosis of PCV as described by Liu et al.[6], [7]
In the present study, mean central choroidal thickness and choroidal thickness at the level of pachyvessel were found to be decreased at the end of the follow up. No statistical significance was associated with change in choroidal thickness in patients treated with and without PDT (either standard or reduced fluence) or number of injections which was comparable to the study by Giridhar et al.[8] Lee et al showed a significant decrease in mean choroidal thickness in PCV patients post anti VEGF therapy.[9] Mean choroidal thickness also decreased at 3 years follow up and was associated with number of intravitreal Ranibizumab injections in yet another study while no significant association between baseline CCT and mean CCT reduction at month 36 was noted by Azuma et al.[10] It has been speculated that an increased extra vascular volume and increased choroidal hyper permeability may be associated with a thicker subfoveal choroid which may show a poor response owing to increased hydrostatic pressure. And these studies show a better response to anti VEGF therapy associated with thinner choroid.[11] Although we cannot directly compare the results due to a different study design, increased propensity of thinner choroid to choroidal ischemia following PDT may be a reason for thicker choroid showing better visual outcomes at the end of five years in the present study.[12]
In our study, BCVA significantly improved from baseline at 5 years although no significant difference was noted in visual gain at the end of one year. Our results were different from that of Hikichi et al. where visual gain was significant at one and two years follow ups compared to the baseline.[13] In our study, the mean BCVA improved from 0.42 to 0.6 log MAR at 5 years which was statistically significant. Improvement in vision was achieved in 42% (21 eyes) eyes, while visual loss occurred in 13 eyes (26%). The deterioration of visual acuity at the end of five years was significantly more in eyes with poor baseline vision <o.60 log MAR (<0.001) and type 1 PCV compared to type 2(<0.001). Our results were similar to another study by Jingyuan Yang et al.[5] who also reported a better outcome in type 2 PCV at 1 year.
In the present study, both the mono therapy and combination therapy showed comparable outcomes which is similar to the results of PLANET study.[4] Unlike the EVEREST study,[3] present study did not show a visual benefit in the combination therapy arm which might be due to PDT done in majority of non responsive cases owing to the retrospective nature of this study. Low fluence and standard fluence PDT showed similar long term outcomes and we speculate the better value of low fluence in PCV management owing to reduced RPE atrophy. Although, RPE atrophy and scarring at the end of five years were more in combination therapy group probably owing to choroidal hypoperfusion caused by PDT, it was not statistically significant. Eyes with RPE atrophy and scarring showed a poor visual outcome.[14] The present study was conducted in real-life practice and variability in treatment protocols with line ant adherence to treatment protocols might have affected our final outcomes.
In the present study, imaging biomarkers that were found to be statistically significant in predicting better response at the end of 5 years follow up were thicker CCT(>232microns) and smaller maximum lesion dimension on ICGA(<1982microns). Lesion size on OCTA has been found to be significantly larger than that determined by ICGA. Being a non invasive tool with easy repeatability and clear network morphology,[15] OCTA is a preferred modality in our current practice. This is an important finding as it has not been well described in other studies available in the literature due to lack of studies focusing on the long term outcomes in the prognosis of PCV. Smaller lesion may show lesser exudative changes and even with exudative changes have shown a favorable response to treatment and has a better clinical course than the larger lesions.[16]
The mean number of injections in the present study group at the end of 5 years follow up was found to be 8.6. This number is considerably less than other similar studies like LAPTOP study[17] which reported a mean of 14.8 injections over 5 years. Even, Hikichi et al showed a mean of 18.2 ranibizumab injections over 6 years.[13] The lower number of mean injections could be due to the poor compliance in the present study group. Majority of anti VEGF switch was done to aflibercept. Efficacy of aflibercept in recurrent and recalcitrant PCV has been established by prior studies.[18]
In the present study, compliance to the treatment for PCV was 86.3%. This compliance can be considered to be low when compared to the study by Taiichi et al where the compliance at the end of 1 year was found to be 95%. The reason for comparatively low compliance in the present study was monetary reasons due to lack of health insurance. In the study by Taiichi et al, the main reasons for interruption were poor physical health and stable fundus findings.[13] The unaffordable treatment population specifically in a developing nation like India can be advised to take suitable health insurance or can be directed to seek social health worker help so as to avoid the interruption in the treatment at later course due to financial reasons.
We found the retrospective nature of the present study and the small number of patients to be the main limitations. Failure to strictly adhere to a specific treatment protocol might also have an influence. Moreover, there was variability in the timing to administer rescue or repeat PDT as it was dependent on the judgement of treating physician and patient willingness. Prospective studies with long term follow-up preferably more than 5 years should be done to further study the detailed.
Conclusion
This real-world data depicts the profile and long term treatment outcomes of PCV patients in our population. The clinical and tomographic patterns are variable with anti VEGF monotherapy being the most preferred treatment. The role of OCTA, low fluence PDT, the need to subtype PCV and the factors predicting better long term outcomes are evident from this study. The superiority of aflibercept and the feasibility of a PRN approach are underlined in this study. In spite of suboptimal compliance this study reveals that nearly half the eyes demonstrated visual gains and anatomic stability.
Source of Funding
None.
Conflicts of Interest
Nil.
References
- LA Yannuzzi, DW Wong, BS Sforzolini, M Goldbaum, KC Tang, RF Spaide. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol 1999. [Google Scholar]
- CM Cheung, TYY Lai, K Teo, P Ruamviboonsuk, SJ Chen, JE Kim. Polypoidal choroidal vasculopathy. Ophthalmology 2021. [Google Scholar]
- TH Lim, TY Lai, K Takahashi, TY Wong, LJ Chen, P Ruamviboonsuk. Comparison of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. JAMA Ophthalmol 2020. [Google Scholar]
- WK Lee, T Iida, Y Ogura, SJ Chen, TY Wong, P Mitchell. Efficacy and safety of Intravitreal AFLIBERCEPT for polypoidal choroidal vasculopathy in the planet study. JAMA Ophthalmol 2018. [Google Scholar]
- J Yang, M Yuan, S Xia, Y Chen. Six-year real-world outcomes of antivascular endothelial growth factor monotherapy and combination therapy for various subtypes of polypoidal choroidal vasculopathy. J Ophthalmol 2019. [Google Scholar] [Crossref]
- R Liu, J Li, Z Li, S Yu, Y Yang, H Yan. Distinguishing polypoidal choroidal vasculopathy from typical neovascular age-related macular degeneration based on Spectral Domain Optical Coherence Tomography. Retina 2016. [Google Scholar]
- A Singh, I Yadav, S Deshmukh, RP Maurya, S Panday. Rare case of exudative retinal detachment in Normotensive HELLP Syndrome: A case report. Ind J Clin Exp Ophthalmol 2015. [Google Scholar]
- G Anantharaman, G Ramkumar, M Gopalakrishnan, A Rajput. Clinical features, management and visual outcome of polypoidal choroidal vasculopathy in Indian patients. Indian J Ophthalmol 2010. [Google Scholar]
- KH Lee, SH Kim, JM Lee, EC Kang, HJ Koh. Peripapillary choroidal thickness change of polypoidal choroidal vasculopathy after anti-vascular endothelial growth factor. Korean J Ophthalmol 2017. [Google Scholar]
- K Azuma, A Okubo, Y Nomura, H Zhou, R Terao, Y Hashimoto. Association between pachychoroid and long-term treatment outcomes of photodynamic therapy with intravitreal ranibizumab for polypoidal choroidal vasculopathy. Sci Rep 2020. [Google Scholar] [Crossref]
- H Kim, SC Lee, KY Kwon, JH Lee, HJ Koh, SH Byeon. Subfoveal choroidal thickness as a predictor of treatment response to anti-vascular endothelial growth factor therapy for polypoidal choroidal vasculopathy. Graefe's Arch Clin Exp Ophthalmol 2015. [Google Scholar]
- PY Lee, KS Kim, WK Lee. Severe choroidal ischemia following photodynamic therapy for pigment epithelial detachment and chronic central serous chorioretinopathy. Jap J Ophthalmol 2009. [Google Scholar]
- T Hikichi, M Higuchi, T Matsushita, S Kosaka, R Matsushita, K Takami. Results of 2 years of treatment with AS-needed ranibizumab reinjection for polypoidal choroidal vasculopathy. Br JOphthalmol 2013. [Google Scholar]
- T Hikichi, H Kitamei, S Shioya. Retinal pigment epithelial atrophy over polypoidal choroidal vasculopathy lesions during ranibizumab monotherapy. BMC Ophthalmol 2016. [Google Scholar]
- CM Cheung, Y Yanagi, M Akiba, A Tan, R Mathur, CM Chan. Improved detection and diagnosis of polypoidal choroidal vasculopathy using a combination of optical coherence tomography and optical coherence tomography angiography. Retina 2019. [Google Scholar]
- M Ratanasukon, P Bhurayanontachai, P Jirarattanasopa. Polypoidal choroidal vasculopathy (PCV): The 4-year review of the real-life treatment experiences. Clin Ophthalmol 2018. [Google Scholar]
- A Oishi, N Miyamoto, M Mandai, S Honda, T Matsuoka, H Oh. Laptop study: A 24-month trial of Verteporfin versus ranibizumab for polypoidal choroidal vasculopathy. Ophthalmology 2014. [Google Scholar]
- SE Lee, JW Jang, SW Kang, KH Park, DW Lee, JH Kim. Intravitreal aflibercept for active polypoidal choroidal vasculopathy without active polyps. Sci Rep 2019. [Google Scholar] [Crossref]
How to Cite This Article
Vancouver
S M, Indurkar A, Iqbal S, V RR, Mathew M, Nair U. How do polypoidal choroidal vasculopathy patients fare long term - A real world data [Internet]. Indian J Clin Exp Ophthalmol. 2023 [cited 2025 Sep 13];9(1):68-74. Available from: https://doi.org/10.18231/j.ijceo.2023.013
APA
S, M., Indurkar, A., Iqbal, S., V, R. R., Mathew, M., Nair, U. (2023). How do polypoidal choroidal vasculopathy patients fare long term - A real world data. Indian J Clin Exp Ophthalmol, 9(1), 68-74. https://doi.org/10.18231/j.ijceo.2023.013
MLA
S, Manoj, Indurkar, Asmita, Iqbal, Sameer, V, Ravi R, Mathew, Mancy, Nair, Unnikrishnan. "How do polypoidal choroidal vasculopathy patients fare long term - A real world data." Indian J Clin Exp Ophthalmol, vol. 9, no. 1, 2023, pp. 68-74. https://doi.org/10.18231/j.ijceo.2023.013
Chicago
S, M., Indurkar, A., Iqbal, S., V, R. R., Mathew, M., Nair, U.. "How do polypoidal choroidal vasculopathy patients fare long term - A real world data." Indian J Clin Exp Ophthalmol 9, no. 1 (2023): 68-74. https://doi.org/10.18231/j.ijceo.2023.013
