Introduction
Optical coherence tomography angiography (OCTA) is a new, noninvasive imaging technique that plays an important role in clinical practice.1, 2 Optical coherence tomography angiography (OCTA) has become an alternative imaging method of the retinal and choroidal vascularity within the past decade. Like ordinary optical coherence tomography (OCT), OCTA provides B-scans of the retina, but OCTA additionally offers rapid, high-resolution, repeated, and accurate visualization of blood flow without injection of a contrast agent, which provides indirect representation of the vascular morphology of the retina and choroid.3 OCTA has been shown to be effective in the detection of CNV.1, 4 Executed studies with different spectral domain OCTA (SD-OCTA) devices (840 nm wavelength, max. 70,000 A-scans/second) for the detection of choroidal neovascularization (CNV) revealed a far-flung sensitivity between 32% and 89.2% and a specificity between 67.7% and 100%.5 Deeper tissue penetration and faster scanning rate with SS-OCTA leads to a better visualization of microvascular retinal structures compared with SD-OCTA. Lumbroso et al. and Muakkassa et al. used OCTA to detect changes in CNV after anti-VEGF treatment.6, 7 Furthermore, with recent developments in equipment and software, OCTA can now measure the size of CNV, so that it can be compared before and after anti-VEGF treatment. Hence we took up this study to evaluate the CNV activity and change in flow patterns of treatment naive exudative AMD by using OCTA. We also analyzed the CVI and CNV flow remodeling response to anti-VEGF therapy as mentioned in methodology.
Materials and Methods
Cross sectional study of 46 treatment naïve eyes with exudative age related macular degeneration (AMD) which had active CNV of type 1, type 2 and mixed type. All patients were enrolled from August 2019 to April 2020. The study was approved by the institutional review board and was in accordance with declaration of Helsinki. Various parameters at baseline and after 3 months follow up were compared. The diagnosis of exudative AMD was made in each case by clinical examination, SD-OCT, fundus fluoresciene angiography, indocyanine green angiography and OCTA (Spectralis HRA; Heidelberg Engineering, Germany) at the baseline. Any patient with previous anti VEGF therapy, Polypoidal choroidal vasculopathy, secondary causes of CNV& eyes in which a good quality OCTA could not be acquired were excluded from the study. We calculated the CVI using the Image J software (version 1.49; National Institutes of Health, Bethesda, MD, USA). CVI gives a measure of the structural changes in the choroid. A central OCT scan passing through the fovea at baseline and after 3 months follow up, was taken for image binarization. We used a protocol proposed by Sonoda et al1 with few modifications described by Agarwal et al.2 OCT image was imported in Image J software. Niblack auto local threshold tool was used to binarize the OCT image. Binarizing an image makes the scleral interface with the choroid sharper. Polygon selection tool was used and 4000µm of choroid under the CNV was selected from retinal pigment epithelium to the choroid sclera junction then added to the region of interest manager. Image was then converted to RGB (red, green, blue) color and color threshold tool was used. Total choroidal area (TCA) and the luminal area (LA) were calculated. CVI was obtained by dividing LA by TCA. (Figure 1)
OCTA was obtained with Spectralis HRA device (Heidelberg Engineering, Germany). Protocol covered 150 x 150area of the macula centre acquiring high resolution scans with 11 µm cuts. The best CNV network area was manually selected for image analysis. 4 types of CNV networks were identified based on previous studies (Figure 2).3, 4, 5, 6 “Sea-fan” pattern showed a peripheral feeder vessel, thin branching and surrounding halo. “Medusa” pattern had a central feeder vessel with circular peripheral anastomosis and peripheral halo. “Indistinct” pattern showed no feeder vessel but had thin branching & surrounding halo. “Pruned vascular tree” showed only the main branch but no thin branching. The total lesion area was taken from the enface image obtained along with the OCTA. Vascular area was calculated from Image J software. OCT was taken using the same device, 200 x 200 degree scan of the macula consisting of 49 horizontal line scans with a spacing of 120µm. Choroid was imaged using enhanced depth imaging (EDI) mode. Subfoveal choroidal thickness (SFCT) was manually calculated using the inbuilt caliper tool of the OCT device. Statistical analysis was performed using SPSS version 16.0. Descriptive analysis of the population’s characteristics was carried out. Results of continuous variables are reported as mean and SD and of categorical variables are reported as count and percentages. The differences between quantitative variables were analyzed using Wilcoxon signed rank test and Paired t test. The differences between categorical variables were analyzed using McNemar’s test. Spearman correlation coefficient was used to find the correlation between the two variables. P-value <0.05 was considered as significant for all the comparisons.
Results
Mean age was 66.34± 7.2 (range 52 to 84 years).A total of 46 eyes of 44 patients with exudative AMD were included and there were 22 men and 22 women. They received a total of 2.75 ± 0.38 injections at the final follow up. The initial BCVA was 0.74 ± 0.56 logMAR and the final BCVA was 0.48 ±0.3 logMAR (p = 0.003). The Subfoveal choroidal thickness at baseline was first 215.39±102.378 µm and at final examination it was 198.42±101.049 (p = 0.07), decrease of 16.97 µm. The baseline CVI was 63.73±5.28% and 64.59±4.87% at final examination (p = 0.754). At baseline CNV showed 8 (17.4%) medusa pattern, 20 (43.5%) seafan pattern, 16 (34.8%) indistinct and 2(4.3%) mixed pattern. At final follow up 6 (13%) medusa, 6(13%) seafan, 28 (60.9%) indistinct and 6 (13%) mixed patterns of CNV were seen. OCT at baseline showed activity in 46 (100%) eyes. Final visit 20 (43.5%) had signs of activity and 26 (56.5%) showed no signs of activity (p=0.01). OCTA at baseline, 36 (78.3%) showed active CNV network and 10 (21.7%) had inactive network. OCTA at final visit, 12 (26.1%) had active network and 34 (73.9) had inactive network (p=0.01). The total CNV lesion area was 4.42 ± 3.16 mm2 at baseline and 3.24 ± 2.21 mm2 at final examination (p = 0.05). The total vascular area of CNV network was 0.66 ± 0.634 mm2at baseline and 0.52 ± 0.44 mm2 at final visit (p= 0.12). As all the eyes were treated with anti- VEGFs we noted intrinsic changes in the flow patterns after 3 months with 60.9% (28/46) eyes showing an indistinct type of network and absence of the feeder vessel in 52.2% (24/46) eyes (p=0.20) (Figure 3). There was a significant change in OCTA activity with 34/46 eyes showing inactive networks after 3 months (p<0.01). Flow patterns in these inactive networks were dominated by indistinct pattern (26), mixed pattern (6) and sea fan pattern (2). The total lesion area correlated positively to the vascular area both at baseline (p=0.005) and after 3 months (p=0.023).
Figure 1
CVI calculation in a patient with exudative AMD. SD-OCT scans were imported into image J software and cropped. A) The area of choroid underneath the CNV was manually selected using the polygon selection tool and the area was saved. B, C, D) Image was then converted
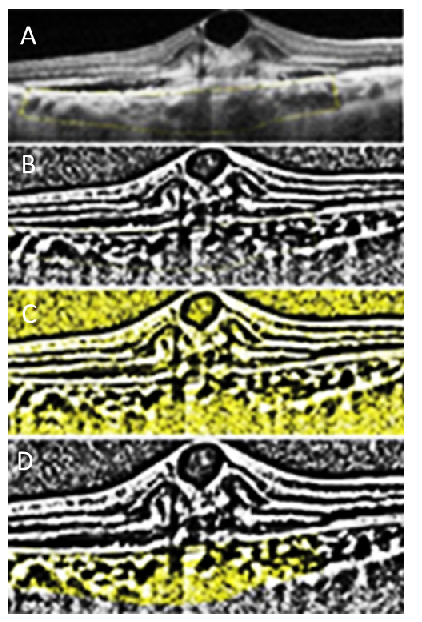
Figure 2
Different CNV patterns in OCTA. A) “Medusa” pattern, showing a central feeder vessel. B) “Seafan” pattern with eccentric feeder vessel. C) “Indistinct” pattern with no feeder vessel. D) “Pruned vascular tree” showing only the main vascular trunk with no thin branches

Figure 3
Showing decrease in both OCT and OCTA activity A) “Indistinct” pattern of CNV & B) SD-OCT showing signs of activity. C) Resolution of the CNV network. D) OCT shows just a uniform sub-retinal hyper reflective material and no intra retinal fluid

Figure 4
A) “indistinct” pattern of CNV on OCTA at baseline. B) Corresponding OCT shows activity with intra retinal fluid and sub retinal mixed reflective material. C) OCTA showing an active network but (D) OCT shows no signs of activity at 3 months follow up

Figure 5
A) “sea fan” pattern of CNV with B) signs of active exudation on OCT at baseline. C) At 3 months the network has decreased in size and D) OCT activity also has decrease

Table 1
Demographic and clinical features
Discussion
In our study, we tried to evaluate the CNV activity and change in flow patterns of treatment naive exudative AMD by using OCTA and analyzing the CVI.CNV flow remodeling in response to anti-VEGF therapy has been describe previously.8, 9 By starting anti-VEGF therapy Lumbroso et al8 have demonstrated vascular remodeling changes which include decrease in the caliber of the smaller branches within 24 hours of injection and a maximum decline in the flow between 6 to 12 days. This phase is followed reproliferation phase 20 to 50 days later. Vascular remodeling phenomenon like arteriogenesis and angiogenesis were studied by Spaide et al in CNV.10 Coscas et al. demonstrated that the different CNV patterns detected on OCTA had some correlation with the treatment decisions my multimodal imaging. 11 We attempted studying the choroidal characteristics may affect the flow through the CNV after anti-VEGF therapy.
In the present study mean SFCT decreased from 215.39 µm to 198.42µm after 3 months showing a decrease of 16.97 µm. SFCT decreases by approximately 4 µm per year during normal aging process.12 The decrease in SFCT in our study was due to the decrease in the disease activity. This decrease in the SFCT has been described to be either due to decreased choroidal hyperpermeability13 or secondary vasoconstriction. 14 Anti-VEGF decreases the blood flow to the CNV network. The pre and post procedure SFCT was not able to predict the final disease activity on OCT and OCTA. So in this study SFCT as a biomarker was inadequate to predict the disease activity pre and post treatment with anti VEGF’s.
The CVI was 63.73 at baseline and 64.59 at the final visit. The change noted was insignificant. In our study there was no correlation between the type of CNV, the pattern of flow in the CNV & CVI at baseline and at final visit. The VEGF levels are more in the area close to the CNV. But the CVI and SFCT show case changes that are present in the choroid spread across the posterior pole in general.15 It is difficult to conclude whether the choroidal changes involve the whole choroid or only the area around the CNV. Unlike the SFCT, CVI is a more stable indicator of choroidal circulation. The shorter follow up time may be the reason why CVI did not show any significant changes in this study.
The present study baseline CNV flow analysis revealed medusa, sea-fan, indistinct and mixed pattern in all the patients (46/46) at the baseline. These networks had the basic features to be classified as “immature” networks.16 We analyzed the CNV lesion area, the CNV vascular area and the type of vascular flow patterns. The CNV flow patterns showed high fluctuation in the total area and vascular area in networks with active flow patterns. Hence the lesion size can be taken as a marker for therapeutic response in treatment naïve age related macular degeneration. OCT surprisingly showed no sign of activity in some eyes which had features of active network (Figure 4). These included medusa head in 2, indistinct in 18 and mixed pattern in 6 eyes. Signs of exudation on OCT were also noticed in eyes which ha inactive networks (Figure 5). The type of flow patterns were analyzed individually for the type of CNV, lesion area and vascular area, which found no significant correlation between them.
OCTA with no CNV network is known to be associated with clinical and OCT inactivity.17 However cyclic CNV variations during follow-up have been studied by Lombroso et al. who mentioned the CNV cycle to be 62 days long.18 Huang et al. showed CNV flow shut down by 2 weeks after injection, then recurrence of flow signals by 4 weeks and OCT activity by 6 weeks,19 Based on the above findings we assume that the CNV networks initially respond to the anti VEGF injections by contraction of lesion area and then later the flow through them changes to develop into mature networks. Anti VEGF therapy initially decreases the choroidal thickness and as the effect wears off the choroidal thickness increases, to thin again as therapy is restarted.20 In this study CVI was analyzed after the development of CNV. Cyclic CNV changes may explain why certain eyes had no OCT features of disease activity even though the OCTA showed an active CNV network. Limitations of this study include its retrospective nature with a small sample size and short follow up period. Angiography was not repeated at the final visit so the leakage from CNV networks with no OCT activity could not be commented. Absence of a control group, manual measurements used in OCT and OCTA analysis were some other limitations. These can be addressed in future studies with larger sample size.
As far as we know this is the first study which has studied the CNV activity combining CVI and flow patterns on OCTA. Choroidal neovascular lesion area is a good biomarker of treatment response. The cyclic variations of CNV have to be kept in mind before making treatment decisions. Using OCT alone in follow up of CNV can miss cases which can reactivate in the future. Cases with OCTA activity and dry OCT scan needs to be followed up more frequently. CVI remains a stable cindicator of choroidal circulation when compared to SFCT. So studies employing CVI can help to further understand the long term vascular remodeling seen in CNV. These new parameters may help in individualized follow-up and treatment of CNV.


