Introduction
Glaucoma is defined as chronic, progressive optic neuropathy caused by a group of ocular conditions, which leads to damage of optic nerve with loss of visual function and often but not invariably associated with increased intraocular pressure.1 Glaucoma affects around 70 million people and is second leading cause of blindness worldwide.2 In India glaucoma is the third most common cause of blindness.3 It is estimated that glaucoma affects around 12 million people aged above 40 years and is responsible for 12.8% of the total blindness in the country,3 of these POAG was estimated to affect 6.48 million people.4
Among the different forms of glaucoma, the POAG is the most common type.5, 6 POAG being a complex disease, the underlying molecular mechanisms are still unclear and it exhibits multifactorial aetiology. Along with elevated IOP, other common risk factors for POAG are age, race, family history, thin cornea, myopia, diabetes, hypertension and oxidative stress.7, 8, 9, 10 Among various risk factors, a positive family history of POAG is considered a major risk factor. Approximately, 16%–22% of first degree relatives of POAG patients develop disease.11, 12
Till date 30 chromosomal loci have been mapped by linkage analysis of which, 17 have been designated from GLC1A to GLC1Q by HUGO (Human genome organization) genome nomenclature committee and mutations in five genes have been reported to be linked to POAG. Among them, myocilin (MYOC; GLC1A), optineurin (OPTN; GLC1E), WD repeat domain36 (WDR36; GLC1G), neurotrophic factor 4 (NTF4; GLC1O) and ankyrin repeat and SOCS box containing 10 (ASB10; GLC1F) have been identified as harbouring disease causative mutations.13, 14, 15, 16, 17 In addition, recent studies show involvement of CYP1B1 in POAG in spite of being a candidate gene for primary congenital glaucoma (PCG).18, 19
MYOC was the first candidate gene mapped in POAG and was identified on 1q21q31. The MYOC gene, located on the GLC1A locus, was initially known as the trabecular meshwork-inducible glucocorticoid response (TIGR) gene. The MYOC gene consists of three exons with two intervening introns and encodes for a transcript of about 2.5kb and is secreted as a 57 kDa glycoprotein composed of 504 amino acids.20 Exon III is largest of the three and is referred to as the olfactomedin like domain due. More than 90% of the mutations reported so far are clustered to exon III of the gene. In human the MYOC is expressed in a number of ocular and non ocular tissues including the TM which exhibit the highest level of expression, followed by the sclera, ciliary body, choroid, cornea, iris, lamina cribrosa, retina and optic nerve. The non ocular tissues include mammary gland, small intestine, thymus, prostate, testis, colon, stomach, thyroid, trachea, bone marrow and brain.21 Till date only trabecular meshwork related MYOC gene mutation identified.
Mutations in MYOC have been reported in different ethnic groups and accounts for 2-5% of POAG cases and around 33% of JOAG cases worldwide.22, 23, 24, 25 Till date, over 100 POAG-associated mutations26 have been identified in the MYOC gene. MYOC gene and its pathogenic variants lead to a toxic gain-of-function due to misfolding and intracellular aggregation of the mutant protein, which leads to reduced aqueous outflow through the trabecular meshwork and schlemm's canal and further causing POAG.27, 28 It is increased in response to elevated IOP, dexamethasone exposure, and other form of trabecular stress, implying that it may have protective and adaptive role in the outflow pathway.
The present study was conducted with primary aim to know prevalence of MYOC mutation in North West Rajasthan population with the help of known MYOC gene mutated primers. The secondary objective of the study was to understand the role of MYOC gene as a cause of primary open angle glaucoma and identification of high risk patients in susceptible population i.e. first degree relatives of established POAG patients.
Materials and Methods
This is a hospital based observational study to identify the involvement of mutations in MYOC gene in disease causation among POAG patients attending OPD in Department of Ophthalmology S.P. Medical College & Associated group of hospitals, Bikaner, India. Cases were studied in terms of clinical examinations, relevant investigations, appropriate treatment and documentation.
All patients having the diagnosis of adult/juvenile-onset POAG, normal tension glaucoma and ocular hypertension of either sex were studied.
The study was performed according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Sardar Patel Medical College, Bikaner, India.
This study was done in two parts. In first part initially 66 consecutive cases of open angle glaucoma giving consent to participate were enrolled in study. In second part of study after obtaining results of genetic study, 16 healthy first degree relatives were taken from patients showing positive and 10 healthy first degree relatives of cases with negative results for pathogenic MYOC gene variants to confirm mutant gene transmission in family. Healthy first degree relatives were those in whom open angle glaucoma was ruled out after complete ocular and systemic examination.
Identification of POAG patients and their first degree relatives involved clinical, ocular and systemic examinations. Each patient underwent a complete ophthalmic examination including best corrected visual acuity, measurement of IOP by applanation tonometry, gonioscopic evaluation of the angle, fundus examination by direct ophthalmoscope after dilatation of pupil, visual field testing with automated perimeter, fundus photography and central corneal thickness estimation by specular microscopy.
For this study inclusion criteria was POAG patients and healthy first degree relatives who give informed consent to participate in the study. Both sporadic and familial POAG cases and those with optic disc and visual field changes suggestive of glaucoma or patients with an IOP greater or less than 21 mm of Hg with treatment were enrolled in the study. Exclusion criteria was patients with a history of ocular inflammation, a history of ocular trauma, angle closure in any quadrant or those not giving informed consent.
After confirmation of diagnosis and obtaining informed consent from the patients and their first degree relatives 2 ml blood was drawn in EDTA vial and send to Multi-Disciplinary Research Units (MDRU) for analysis.
Analysis of the MYOC gene
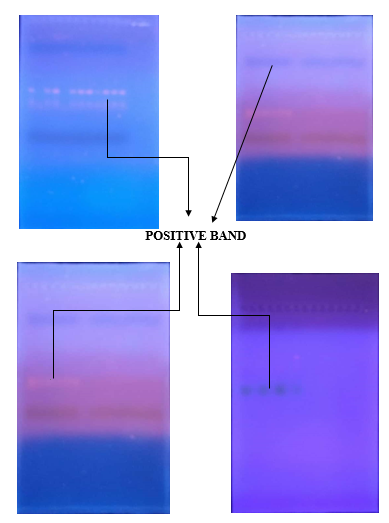
Genomic DNA was isolated from blood samples of patients and healthy first degree relatives using genomic extraction kits and quantification of DNA was done at 260/280 nm on spectrophotometer then quantified DNA was used for further analysis. Twelve primer sets were designed to amplify the gene. (Table 1). PCR amplifications were carried out in 25 µl reaction volumes containing about 25 nMol of genomic DNA, 0.5 µl of each primer dilute to 10 pmol in PCR master mix. Amplification was carried out under the following conditions: initial denaturation 94 °C for 5 min, followed by 30 cycles of denaturation 94 °C for 30 s, annealing for 30 s, extension 72 °C for 30 s, followed by a final extension at 72 °C for 2 min and finally the PCR products were analyzed by electrophoresis in 1.2% agarose gel.
Table 1
Primer sequences used to amplify candidate genes in the study
Results
66 POAG patients, comprising 49 sporadic cases and 17 patients with a family history of POAG were enrolled in this study. Among these 66 patients, 55 were Adult onset POAG, 5 were Juvenile onset POAG, 4 were NTG and 2 were Ocular Hypertension. Age range of study population was 23 years to 78 years, with a mean age (±standard deviation) of 56.77±10.83years. These patients were not known to have any other eye disorder.
In the first part of study peripheral blood sample of 66 patients was examined to detect the presence of nucleotide changes by PCR and electrophoresis.
Out of 66 patients, we detected pathogenic MYOC gene variant in 16 open angle glaucoma cases. Of these 16 cases, pathogenic MYOC gene variant was found in 12 adult onset POAG cases, 3 in juvenile onset POAG and 1 in ocular hypertension case. No pathogenic MYOC gene variant was found in NTG cases. Out of 12 pathogenic MYOC gene variants, only one gene variant MYOC 4F/MYOC 4R was found in all 16 positive cases of open angle glaucoma.
Table 2
Pathogenic MYOC gene variants distribution in defined study group
6 cases were related to each other (all were first degree relatives) out of total 16 positive cases for pathogenic MYOC gene variant. Out of 17 cases of familial glaucoma, 7 cases were positive for mutant myocilin gene variant in which 4 cases (7.37%) were adult onset POAG, 3 cases JOAG and 1 NTG.
Table 3
Pathogenic MYOC gene variants distribution in healthy first degree relatives group
Mutated PCR primer MYOC 4F/MYOC 4R indicated a base change (1109C->T) in exon 3 that would cause a missense mutation (Pro370Leu).
The change C>T transition at nucleotide 1109, results in change from proline (a polar amino acid) to hydrophobic leucine molecule. Moreover, it has been elucidated that proline has a side chain that inhibits (α helix) formation and fits poorly in α helix conformation, while leucine is one of the good α helix formers.29 The severe nature of disease in patients with this mutation indicates that the loss of proline at this position may acutely affect the normal function of the protein.
Mean age of cases positive for Pro370Leu mutation was 52.937±12.363 which was found to be lower than those patients who were negative for pathogenic MYOC gene variant (56.772±10.838). Hence age of presentation was earlier in positive pathogenic MYOC gene variants cases.
Mean IOP of cases positive for Pro370Leu mutation was 19.796±3.277 mm hg higher than cases negative for any pathogenic MYOC gene variants (18.982±4.227 mm hg) indicating severity of glaucoma in Pro370Leu mutation cases. Mean CCT of cases positive for Pro370Leu mutation was 507.774±20.980 µm lower than cases negative for any pathogenic MYOC gene variants (510.535±28.192). Mean Axial length of cases positive for Pro370Leu mutation was (23.549±0.574 mm) slightly higher than cases negative for any pathogenic MYOC gene variants (23.325±1.097 mm).
3 cases of JOAG which were showing Pro370leu changes on exon 3 of MYOC have early age of onset with higher mean IOP (22.12±2.08) and axial length(23.73±0.54) and lower CCT (499.8±10.30) and severe visual field changes in perimetry compared to adult onset POAG. These all 3 cases of JOAG were showing poor response to multi drug treatment and surgical intervention.
Table 4
Parameters distribution in defined myocilin gene study population
Most positive cases showed moderate to severe visual field defects like superior and inferior paracentral scotoma, siedel scotoma, superior and inferior arcuate scotoma and some cases showed normal visual field. Our study showed moderate to severe visual field defects in positive Juvenile open angle glaucoma cases
In second part of our study we included 26 healthy first degree relatives of POAG cases. Out of 26 healthy first degree relatives, 4 cases were positive for pathogenic MYOC gene variant. And all the 4 cases were related to pathogenic MYOC gene positive open angle glaucoma patients.
All first degree relative which were positive for pathogenic MYOC gene variants had family history of POAG.
All the four first degree relatives did not show any signs of POAG, which was showing incomplete penetrance and variable expessibility of pro370leu MYOC mutation in first degree relatives.
In healthy first degree relatives of positive cases all the determinants like IOP, CCT, and AL were 15.15±2.333 mm Hg, 539.633±18.092µm, 22.167±0.982mm respectively. Values show that healthy relatives of positive cases had lower IOP and AL and higher CCT than positive/negative MYOC open angle glaucoma cases. In the first degree relatives of negative cases values were 14.57±2.103mm Hg, 527.95±16.073µm, 22.595±0.830mm. These values shows that first degree relatives of negative cases had lower IOP and AL and higher CCT than positive/negative MYOC open angle glaucoma cases.
Discussion
The study on the North West Indian POAG cohort (n=66) led to identification of 1 pathogenic MYOC gene variant among 16 patients (24.24%) which is much higher than studied elsewhere in India and other countries regarding prevalence of MYOC gene mutation in POAG cases (2%–5%). This could be due to geographic and ethnic variation and most probably due to high MYOC gene mutation prevalence in this area. It could also be due to inadequate data and lack of studies on the subject from other parts of country. Further high positive rate could also be due to diagnostically proven and confirmed cases recruited in our study.
In our study frequency of transmission of pathogenic MYOC gene variants in first degree relatives was 25%, which was similar to other studies done worldwide (16-22%).
Till date, involvement of MYOC in POAG has been examined mostly in eastern and southern parts of India only. A complete list of mutations in the MYOC gene identified in the Indian population is listed in the Indian Genetic Disease Database.30 Among the identified genetic defects, Gln48His is the most prevalent mutation detected in the Indian population. The most prevalent Gln368Stop mutation may give rise to milder POAG presentation with late onset.31 The Pro370Leu mutation are responsible for the most severe glaucoma phenotypes with early onset.31
There has been a lack of discernible phenotype in both MYOC heterozygous and MYOC null mice.32 Also, absence of POAG in carriers with MYOC homozygous mutations, in contrast to disease phenotype in carriers of heterozygous mutations,33 points toward the fact that disease-causing mutations in humans are likely to act by gain of function.
An early age of disease onset and rapid progression of the disease has also been observed in some patients with an apparently normal MYOC gene. These observations indicate the possible role of other genes in causation of POAG. Mutations in presently known genes account for only a low percentage of all POAG cases (less than 10%).34 It has been found that mutations in MYOC account for more than 10% of dominant juvenile open-angle glaucoma cases and approximately 3% to 4% of unselected adult onset POAG.35 Hence, more extensive genetic studies are required to identify other genes involved and to understand the molecular mechanisms of the disease. Most cases of primary open angle glaucoma are complex and multi-factorial,36 hence the role of epigenetic processes such as DNA methylation and histone modifications along with small interfering RNA cannot be ruled out and have to be addressed to better understand the complete mechanism of disease onset and prognosis.
Conclusion
In conclusion, the screening of MYOC in a representative cohort of 66 POAG individuals and 26 healthy first degree relatives from the ethnically admixed population in North West Rajasthan revealed 16 individuals (24.24%) and 4 healthy related controls(25%) with disease causative mutations pro370leu in exon 3of MYOC gene.
This study is unique since all variants of open angle glaucoma like adult onset POAG, JOAG, OHT and NTG have been enrolled in this study and a comparison of various key diagnostic elements used for assessment of POAG like IOP, CCT, AL, gonioscopy, VFA and pathogenic MYOC gene variants have been evaluated in single study. Prevalence of pathogenic MYOC gene in POAG has been detected and presence of the mutant gene identified in first degree relatives. Transmission rate of the pathogenic MYOC gene in siblings and kins has also been evaluated in POAG.
These results provide evidence to prove that DNA screening is a useful method with high specificity and sensitivity for early detection of the at-risk individual in a glaucoma pedigree. Understanding the molecular basis of glaucoma is important to several aspects of glaucoma diagnosis and management.37 Identifying novel pathways could be used to design more specific and effective therapies. Thus, gene screening can be used for pre-symptom diagnosis and forewarning in familial open-angle glaucoma patients, especially in pedigrees with early-onset.