Introduction
Retinal vein occlusion (RVO), is the second most common cause of retinal vascular disease after diabetic retinopathy, and a significant cause of unilateral and painless loss of vision.1, 2, 3 Based on the localization of venous occlusion, the most frequent types are branch RVO (BRVO), defined as occlusion of a branch of the retinal vein system, and central RVO (CRVO), defined as occlusion located in the central retinal vein. 4, 5 RVO is a significant cause of vision loss with overall incidence of 0.21% among patients of age ≥40 years.6
The prevalence of RVO ranges from 0.7 to 1.6%.7 An estimated 16 million people globally develop RVO, of which BRVO comprises 80% of cases.8 BRVO is more common than CRVO, 9 and affects approximately 1% of the population. The estimated prevalence of BRVO ranges from 0.6 to 1.1%.10 It can cause severe vision loss due to macular edema, retinal neovascularization, and retinal detachment.11 Arterial stiffness is main pathogenic mechanism for the development of BRVO, that can cause venous compression in the common adventitial sheath.4, 7 aging, cardiovascular diseases, smoking, and hypertension are the main risk factors for BRVO.12
Treatment for managing macular edema with BRVO include macular grid laser photocoagulation and anti-vascular endothelial growth factor (VEGF) inhibitors. The treatment of retinal vascular diseases, including BRVO, has been improved with the introduction of intravitreal pharmacotherapy. Anti-VEGF therapy has now become the treatment of choice for macular edema due to BRVO. Many multicenter studies have shown that anti-VEGF treatment to be effective in reducing intra retinal fluid (IRF) and retinal hemorrhages, associated with a statistically significant improvement in visual acuity.13, 14, 15 Ranibizumab, dexamethasone implants, off-label bevacizumab, and triamcinolone have demonstrated efficacy in the therapy of macular edema due to RVO and significantly expanded treatment options, becoming the standard of care.14, 15, 16, 17
In the pathogenesis of BRVO, VEGF plays an important role, with upregulated expression by hypoxia and a number of other stimuli. In BRVO patients, the VEGF concentration in the ocular fluid is increased, that correlates with the severity of macular edema.5, 18
Many anti-VEGF agents have been widely used for treating macular edema due to BRVO, including ranibizumab. Ranibizumab binds and inhibits VEGF, an important driver of macular edema in BRVO. The efficacy of intravitreal ranibizumab for treating macular edema in patients with BRVO has been established in many studies.15 The pivotal phase III BRVO.19 study helped the approval of ranibizumab for the treatment of visual impairment due to macular edema secondary to BRVO. Ranibizumab has been approved in the USA (2010) and the European Union (2011) for the treatment of visual impairment due to macular edema due to RVO. Razumab® (the world’s first biosimilar of ranibizumab by Intas Pharmaceuticals Ltd.) was approved by the Drug Controller General of India in February 2015 for the treatment of wet age-related macular degeneration, diabetic macular edema, macular edema following RVO, and visual impairment because of choroidal neovascularization secondary to pathological myopia. The efficacy and safety of ranibizumab in the treatment of BRVO has been established in many prospective, randomized, double-masked clinical trials. The efficacy and safety of Razumab® has been demonstrated in a prospective study in Indian patients with chorioretinal vascular diseases including BRVO.20 Hence, present study was planned “To Study Early And Late Effect Of Intravitreal Injection Ranibizumab on Cystoid Macular Edema Due To Branch Retinal Vein Occlusion ” with following objectives in our setting.
Aim of The Study
Aim of this study "To study early and late effect of intravitreal injection Ranibizumab on Cystoid Macular Edema(CME) due to branch retinal vein occlusion ".
Material and Methods
This is an observational study included 25 eyes in 25 patients(≥ 18years) with cystoid macular edema because of Branch retial vein occlusion in our tertiary care hospital. The treatment procedure was intravitreal ranibizumab at the dose of 0.50 mg in 0.05 ml. An observational study including has been concluded in which improve in visual acuity and reduction in central macular thickness has been compared in patients with cystoid macular edema presented within and after 4 weeks of onset of BRVO.
Exclusion criteria
Presence of any ocular disease that might cause interpretation of study results, compromise VA, affect macular oedema or require medical or surgical intervention in the study eye during study period;
Any active ocular infection or inflammation in either eye at the time of screening or baseline visit;
Uncontrolled glaucoma (intraocular pressure (IOP) =30 mmHg on medication) at the screening or within 6 months before the baseline visit, or neovascularization of the iris or neovascular glaucoma in either eye;
Past episode of BRVO in the study eye;
Past treatment with any anti-angiogenic drug including any anti-VEGF drug in either eye, within 3 months before the baseline visit;
Intra-/periocular corticosteroids within 3 months before the screening visit, past pan-retinal laser photocoagulation,˂3months before visit or scheduled within the next 3 months following baseline visit, past focal/grid laser photocoagulation, ˂4 months before the baseline visit or any intraocular corticosteroid implants in the study eye;
Treatment with any systemic anti-VEGF drug, ˂6 months before the baseline visit;
History of stroke; or
Uncontrolled blood pressure (systolic value of >160 mmHg or diastolic value of >100 mmHg) at the screening.
Examination
An informed consent was taken from all patients included in the study and underwent the following pre-operative evaluation
Visual acuity assessment,
Intra-ocular pressure (IOP
Slit lamp examination for anterior Segment
Fundus examination-Direct and Indirect ophthalmoscopy
Optical coherence tomography (OCT
Technique of injecting intravitreal Ranibizumab
The eye was topically anesthetized and a povidone iodine (10%) cleaning was performed on the lids and lashes. A Sterile speculum was placed between the eyelids. Povidone iodine (5%) drops were then instilled on the ocular surface three times. Additional topical anesthetisia was achieved by instilling 4% lidocaine. Ranibizumab (0.05 ml; 0.50mg) in an insulin syringe with a 30-gauge needle was then injected through the pars plana into the vitreous cavity through the sclera 3 to 4 mm posterior to the limbus at infero-temporal quadrant. Post injection light perception was assessed and intraocular pressure(IOP) was monitored. After the injection, the patient was advised to apply topical antibiotics to the injected eye 4 times a day for 3 days.
Follow-up was done at 1st day , 1 week , 2 week, and 4 week post-injection
Observation
Table 1
Number of patients presented within 4 week and after 4 week of onset of BRVO
|
Gender |
Within 4 weeks |
After 4 weeks |
|
Male |
6 |
7 |
|
Female |
4 |
8 |
Table 2
Change in BCVA at 4 week followingintravitreal Ranibizumab in patients presented within 4 week and after 4 week of onset of BRVO.
|
Improved BCVA |
No. of pts within 4 weeks |
Percentage |
No. of pts after 4 weeks |
Percentage |
|
1 Line |
2 |
20% |
5 |
33.33% |
|
2 Lines |
1 |
10% |
7 |
46.67% |
|
3 Lines |
2 |
20% |
1 |
6.67% |
|
4 Lines |
4 |
40% |
2 |
13.33% |
|
5 Lines |
1 |
10% |
- |
- |
Result
Baseline characteristics
A total of 25 eyes from 25 consecutively consented patients received an intravitreal injection of Ranibizumab. All 25 patients completed follow-up visit.
These 25 patients had a mean age of 58 53 year.
Out of 25 patients with BRVO, 13 were male and 12 female.
Out of 25 patients,10 patients were presented within 4 weeks and 15 patients after 4 weeks of onset of BRVO.
Of the 25 eyes, none had received prior therapy like laser photocoagulation or intravitreal triamcinolone before receiving intravitreal Ranibizumab.
The baseline mean systolic and diastolic BP value were 139 22 and 85 27mmHg respectively.
Safety
A total of 25 injection of Ranibizumab was performed in 25 eyes and follow-up was done at 1st day, 1 week, 2 weeks and 1 month post- injection.
The injection was well tolerated in all patients. There were no episode of inflammation or severe vision loss. There were no case of endophthalmitis, uveitis, ocular toxicity, retinal detachment or lens damage.
Blood pressure was monitored before injection and each follow-up and no significant elevation was observed over course of study. Known hypertensive continued their antihypertensive treatment with no new antihypertensive additions.
During follow-up, there were no thromboembolic events like cerebrovascular accidents, transient ischaemic attacks, myocardial infarction or peripheral vascular disease.
During follow-up, IOP was stable. No topical anti-glaucoma therapy was needed. Since injected volume of Ranibizumab was very small (0.05 ml) ,no paracentesis was needed.
Visual acuity outcomes
Within 1 week after Ranibizumab injection improvement in visual acuity were observed and these significant changes continued through 4 weeks.
At week 4, out of 10 patients of BRVO who presented within 4 weeks of onset of BRVO, 2 patients showed 1 lines improvement, 1 patient 2 lines, 2 patients 3 lines, 4 patients 4 lines and 1 patient showed 5 lines improvement in visual acuity.
Out of 15 patients of BRVO who presented after 4 weeks of onset of BRVO , 5 patients showed 1 line improvement, 7 patients 2 lines,1 patient 3 lines and 2 patients showed 4 lines improvement in visual acuity.
Optical coherence tomography outcomes
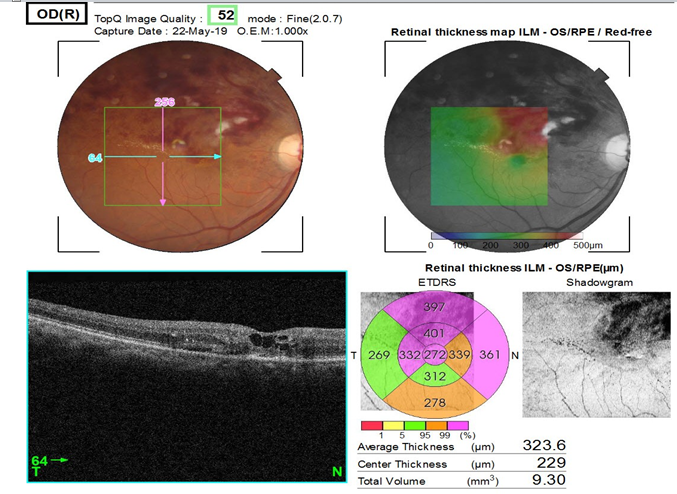
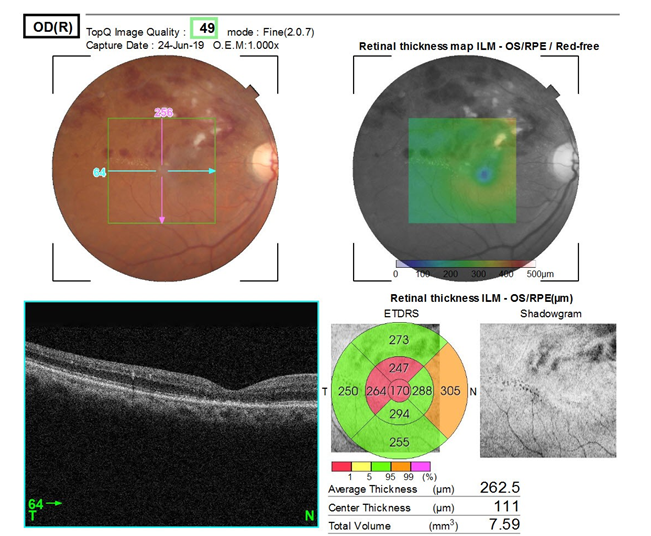
Mean decrease in central macular thickness in 10 patients of BRVO who presented within 4 weeks of onset of BRVO was 195 µm and in 15 patients who presented after 4 weeks of onset of BRVO was 125 µm.
Discussion
Intravitreal Ranibizumab treatment was associated, on average, with rapid gain of visual acuity in patients who presented within 4 weeks of onset of Branch Retinal Vein Occlusion (BRVO) compare to presented after 4 weeks of onset of BRVO.
This was paralled by a rapid decrease in central macular thickness in patients who presented within 4 weeks of onset of Branch retinal vein occlusion (BRVO) compare to presented after 4 weeks of onset of BRVO.
Visual acuity
In our study, 25 patients were of BRVO
At the end of 4 weeks, out of 25 patients of BRVO, 10 patients presented within 4 weeks of onset of BRVO and showed improvement in BCVA of 1 line in 2 patients (20%), 2 lines in 1 patients (10%), 3 lines in 2 patients (20%), 4 lines in 4 patients (40%) and 5 lines in 1 patients (10%).
Out of 25 patients of BRVO, 15 patients presented after 4 weeks of onset of BRVO and showed improvement in BCVA of 1 line in 5 patients(33.33%), 2 lines in 7 patients (46.67%),3 lines in 1 patients (6.67%), 4 lines in 2 patients (13.33%) and none showed further improvement.
Central Macular Thickness(CMT)
In BRVO, patients who presented within 4 weeks of onset of BRVO showed mean reduction in CMT was 195 µm and who presented after 4 weeks of onset of BRVO, showed mean reduction in CMT was 125 µm(P=0.040).
Conclusion
In the present prospective, observational study, an intravitreal Ranibizumab injection 0.50 mg in 0.05 ml for cystoid macular edema due to Branch retinal vein occlusion was safe and tolerated with improvement in visual acuity and reduction in central macular thickness on OCT.
Results of my study are comparative to the result of previous studies. The difference between the baseline values and during follow-up and maximum BCVA and decreased CMT was significant.
Gain in VA at 4 weeks was more in patients presented within 4 weeks of onset of BRVO as compare to present after 4 weeks of onset of BRVO.
Similarly, decrease in CMT at 4 weeks was more in patients presented within 4 weeks of onset of BRVO as compare to presented after 4 week of onset of BRVO.
Thus, intravitreal Ranibizumab is more effective in patients presented early than late in improving BCVA and decreasing CMT.